
The total number of basic groups in the following form of lysine is:
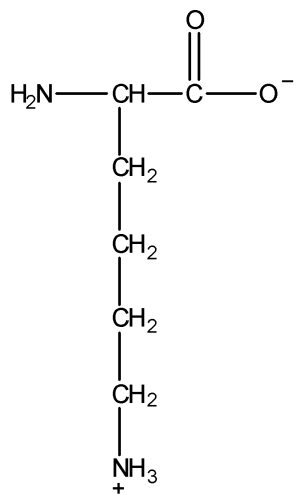
A) 0
B) 1
C) 2
D) 3
E) 4
F) 5
G) 6
H) 7
I) 8
J) 9

Answer
578.4k+ views
Hint: Recall the definition of a base. According to the Bronsted-Lowry acid-base theory, a base is a substance that can accept hydrogen cations (${H^ + }$). Examine the given structure of lysine to find the groups that can easily accept ${H^ + }$ ions and that will be the basic groups.
Complete step by step answer:
The given structure of lysine is as follows:
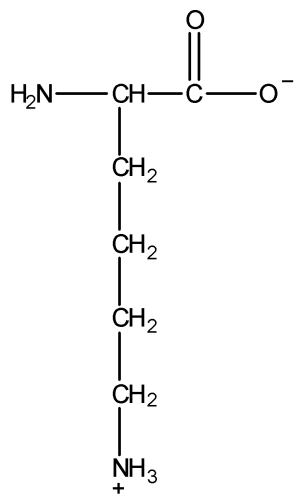
To find basic groups, first we should have knowledge of a base. There are many theories which define base but the general theory which is required to answer this question is Bronsted-Lowry acid-base theory. According to the Bronsted-Lowry acid-base theory, a base is a substance that can accept hydrogen cations (${H^ + }$), also known as protons. So, the basic group in the structure of lysine would be the one which can accept ${H^ + }$ ions easily.
In the given structure of lysine, $ - COO$ group has a negative charge on one oxygen atom, and hence this oxygen atom can easily accept a hydrogen cation (${H^ + }$) to become more stable OH. Thus, $ - COO$ group will try to accept ${H^ + }$ ion to become more stable $ - COOH$. Hence, we can say that $ - COO$ group is a basic group in the given structure of lysine.
Now, there is a $ - N{H_2}$ group in the structure. We all know that nitrogen has a lone pair on it. $ - N{H_2}$ group is an incredibly strong base because it can accept a proton or ${H^ + }$ ion to become $N{H_3}$ (ammonia) which is more stable. Hence, $ - N{H_2}$ group is another basic group.
Hence, there are two basic groups in the given structure of lysine.
So, the correct answer is “Option C”.
Note: Lysine (symbol: Lys) is an $\alpha $-amino acid and is basic in nature. It is a basic amino acid because it has more number of basic groups than acidic groups. Also, lysine is one of the essential amino acids since the human body cannot synthesize it.
Complete step by step answer:
The given structure of lysine is as follows:

To find basic groups, first we should have knowledge of a base. There are many theories which define base but the general theory which is required to answer this question is Bronsted-Lowry acid-base theory. According to the Bronsted-Lowry acid-base theory, a base is a substance that can accept hydrogen cations (${H^ + }$), also known as protons. So, the basic group in the structure of lysine would be the one which can accept ${H^ + }$ ions easily.
In the given structure of lysine, $ - COO$ group has a negative charge on one oxygen atom, and hence this oxygen atom can easily accept a hydrogen cation (${H^ + }$) to become more stable OH. Thus, $ - COO$ group will try to accept ${H^ + }$ ion to become more stable $ - COOH$. Hence, we can say that $ - COO$ group is a basic group in the given structure of lysine.
Now, there is a $ - N{H_2}$ group in the structure. We all know that nitrogen has a lone pair on it. $ - N{H_2}$ group is an incredibly strong base because it can accept a proton or ${H^ + }$ ion to become $N{H_3}$ (ammonia) which is more stable. Hence, $ - N{H_2}$ group is another basic group.
Hence, there are two basic groups in the given structure of lysine.
So, the correct answer is “Option C”.
Note: Lysine (symbol: Lys) is an $\alpha $-amino acid and is basic in nature. It is a basic amino acid because it has more number of basic groups than acidic groups. Also, lysine is one of the essential amino acids since the human body cannot synthesize it.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




