
The sulphur molecule \[{{S}_{8}}\] can be represented as:
a.) Cubical structure
b.) Spherical structure
c.) Tetrahedral structure
d.) W shaped ring structure
Answer
582.9k+ views
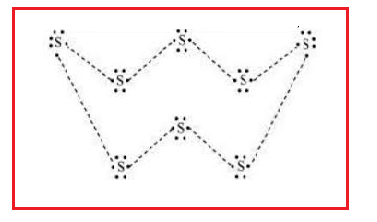
Hint: Eight sulphur atoms form a puckered ring or crown structure to form an eight-atom molecule. Sulphur has six electrons in its outermost shell and requires two electrons to complete its octet state.
Complete step by step answer:
Sulphur has six electrons in its outermost shell and requires two electrons to complete its octet state. So, each sulphur atom shares two electrons, 1 with each adjoining sulphur atom
The atomic number (Z) of sulphur is sixteen and its electronic configuration is 2, 8, 6. The sulphur atom has six valence electrons. The chemical formula of sulphur molecules is \[{{S}_{8}}\] each sulphur atom is linked to similar atoms on either side by single covalent bonds and thus, completes its octet. The molecule is in the form of a ring also represented by crown shape.
So, the correct answer is “Option D”.
Note: Sulphur is abundant, multivalent, and non-metallic. Under normal conditions, Sulphur atoms form cyclic octatomic molecules with a chemical formula \[{{S}_{8}}\]. Elemental sulphur is a bright yellow crystalline solid at room temperature.
The most commonly encountered form of sulphur is the orthorhombic polymorph of \[{{S}_{8}}\], which adopts a puckered ring – or "crown" – structure. Two other polymorphs are known, also with nearly identical molecular structures. In addition to \[{{S}_{8}}\], sulphur rings of 6, 7, 9–15, 18 and 20 atoms are known. At least five allotropes are uniquely formed at high pressures, two of which are metallic. It is used in the manufacture of sulphuric acid, which in turn goes into fertilizers, batteries and cleaners. It's also used to refine oil and in processing ores. Pure sulphur has no smell.
Complete step by step answer:
Sulphur has six electrons in its outermost shell and requires two electrons to complete its octet state. So, each sulphur atom shares two electrons, 1 with each adjoining sulphur atom
The atomic number (Z) of sulphur is sixteen and its electronic configuration is 2, 8, 6. The sulphur atom has six valence electrons. The chemical formula of sulphur molecules is \[{{S}_{8}}\] each sulphur atom is linked to similar atoms on either side by single covalent bonds and thus, completes its octet. The molecule is in the form of a ring also represented by crown shape.
So, the correct answer is “Option D”.

Note: Sulphur is abundant, multivalent, and non-metallic. Under normal conditions, Sulphur atoms form cyclic octatomic molecules with a chemical formula \[{{S}_{8}}\]. Elemental sulphur is a bright yellow crystalline solid at room temperature.
The most commonly encountered form of sulphur is the orthorhombic polymorph of \[{{S}_{8}}\], which adopts a puckered ring – or "crown" – structure. Two other polymorphs are known, also with nearly identical molecular structures. In addition to \[{{S}_{8}}\], sulphur rings of 6, 7, 9–15, 18 and 20 atoms are known. At least five allotropes are uniquely formed at high pressures, two of which are metallic. It is used in the manufacture of sulphuric acid, which in turn goes into fertilizers, batteries and cleaners. It's also used to refine oil and in processing ores. Pure sulphur has no smell.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




