
The structure of white phosphorus is:
(A) square planar
(B) pyramidal
(C) tetrahedral
(D) trigonal planar
Answer
582k+ views
Hint: White phosphorus is allotropy phosphorus when a substance exists in more than one forms, they are called allotropes.
Phosphorus exists as white phosphorus, Red phosphorus and black phosphorus.
Step by step answer: White phosphorus exists as tetra phosphorus $({P_4})$one molecule of phosphorus contains four atoms.
They form tetrahedral structures.
The molecule consists of six single p-p bonds
Due to tetrahedral arrangement, the ring has strain and thus instability.
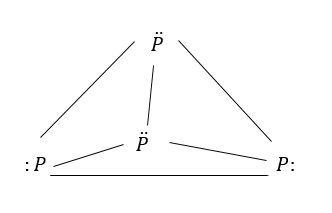
Each phosphorus atom has lone pair of electron
Atomic number of phosphorus is $15.$
Its electronic configuration is, $[Ne]3{s^2}3{p^3}$
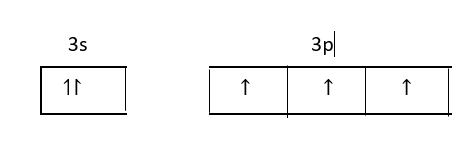
Three unpaired electrons of 3p orbital form a covalent bond with three phosphorus atoms and electrons of 3s orbitals remain as lone pairs of electrons.
Hence option C is correct.
Additional Information:
White phosphorus is unstable due to strain in angle of bonds.
It is highly inflammable and self-igniting
It is very toxic and causes liver damage or ingestion and posy jaw on inhalation.
Samples are generally coated with phosphorus pentoxide which consist of ${P_4}{O_{10}}$tetrahedral with oxygen inserted between phosphorus atoms and at their vertices.
The combustion of phosphorus gives phosphorus oxide.
${P_4} + S{O_2} \to {P_4}{O_{10}}$
White phosphorus can be produced using several different methods.
Phosphorus rock is heated in the presence of carbon and silica. Elemental phosphorus is liberated as vapor and can be collected under phosphorus acid.
Note: White and Red phosphorus are allotropes of phosphorus.
White phosphorus is unstable while Red phosphorus is stable.
White phosphorus is converted into thermodynamically more stable Red phosphorus
Phosphorus exists as white phosphorus, Red phosphorus and black phosphorus.
Step by step answer: White phosphorus exists as tetra phosphorus $({P_4})$one molecule of phosphorus contains four atoms.
They form tetrahedral structures.
The molecule consists of six single p-p bonds
Due to tetrahedral arrangement, the ring has strain and thus instability.

Each phosphorus atom has lone pair of electron
Atomic number of phosphorus is $15.$
Its electronic configuration is, $[Ne]3{s^2}3{p^3}$

Three unpaired electrons of 3p orbital form a covalent bond with three phosphorus atoms and electrons of 3s orbitals remain as lone pairs of electrons.
Hence option C is correct.
Additional Information:
White phosphorus is unstable due to strain in angle of bonds.
It is highly inflammable and self-igniting
It is very toxic and causes liver damage or ingestion and posy jaw on inhalation.
Samples are generally coated with phosphorus pentoxide which consist of ${P_4}{O_{10}}$tetrahedral with oxygen inserted between phosphorus atoms and at their vertices.
The combustion of phosphorus gives phosphorus oxide.
${P_4} + S{O_2} \to {P_4}{O_{10}}$
White phosphorus can be produced using several different methods.
Phosphorus rock is heated in the presence of carbon and silica. Elemental phosphorus is liberated as vapor and can be collected under phosphorus acid.
Note: White and Red phosphorus are allotropes of phosphorus.
White phosphorus is unstable while Red phosphorus is stable.
White phosphorus is converted into thermodynamically more stable Red phosphorus
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




