
The structure of paracetamol is:
(A)
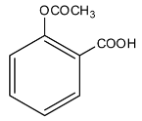
(B)
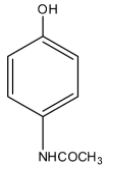
(C)
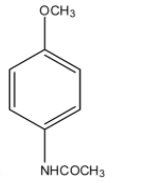
(D)
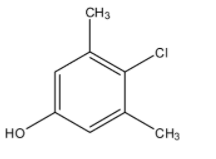




Answer
579.9k+ views
Hint: Paracetamol is a famous drug that is a non-narcotic or non-addictive analgesic that is used to reduce or abolish the pain without causing the impairment of consciousness. Paracetamol is the common name, its chemical name is 4-Acetamidophenol.
Complete step by step solution:
If we want to reduce the pain of any body part or to reduce the body temperature we usually take paracetamol, so paracetamol is a famous drug that is a non-narcotic or non-addictive analgesics that is used to reduce or abolish the pain without causing the impairment of consciousness. It doesn't also cause any mental confusion, incoordination, paralysis, or any kind of disturbance in the nervous system. Paracetamol is the common name, its chemical name is 4-Acetamidophenol. So in the structure of the paracetamol, there is a phenol molecule and at the para position, there is an acetamide group. So the structure of paracetamol is given below:
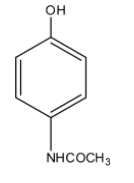
So the chemical formula of paracetamol is ${{C}_{8}}{{H}_{9}}N{{O}_{2}}$ and the molar mass of paracetamol is 151.165 g/mol. The melting point of paracetamol is ${{169}^{\circ }}C$. The boiling point of paracetamol is ${{420}^{\circ }}C$. It is not easily soluble in water.
Sometimes paracetamol is mixed with other chemicals to form a drug that can be used to treat a mild cold.
So the correct answer is an option (B).
Note: Generally paracetamol is safe to take, but its daily dose for an adult can cause severe problems. Mainly it can affect the working of the liver and a higher dosage of paracetamol can cause liver failure.
Complete step by step solution:
If we want to reduce the pain of any body part or to reduce the body temperature we usually take paracetamol, so paracetamol is a famous drug that is a non-narcotic or non-addictive analgesics that is used to reduce or abolish the pain without causing the impairment of consciousness. It doesn't also cause any mental confusion, incoordination, paralysis, or any kind of disturbance in the nervous system. Paracetamol is the common name, its chemical name is 4-Acetamidophenol. So in the structure of the paracetamol, there is a phenol molecule and at the para position, there is an acetamide group. So the structure of paracetamol is given below:

So the chemical formula of paracetamol is ${{C}_{8}}{{H}_{9}}N{{O}_{2}}$ and the molar mass of paracetamol is 151.165 g/mol. The melting point of paracetamol is ${{169}^{\circ }}C$. The boiling point of paracetamol is ${{420}^{\circ }}C$. It is not easily soluble in water.
Sometimes paracetamol is mixed with other chemicals to form a drug that can be used to treat a mild cold.
So the correct answer is an option (B).
Note: Generally paracetamol is safe to take, but its daily dose for an adult can cause severe problems. Mainly it can affect the working of the liver and a higher dosage of paracetamol can cause liver failure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




