
The structure of isoprene is:
A. \[{\text{C}}{{\text{H}}_{\text{3}}} - {\text{CH}} = {\text{C}} = {\text{C}}{{\text{H}}_{\text{2}}}\]
B. \[{\text{C}}{{\text{H}}_{\text{3}}} - {\text{CH}} = {\text{C}}{{\text{H}}_{\text{2}}}\]
C.
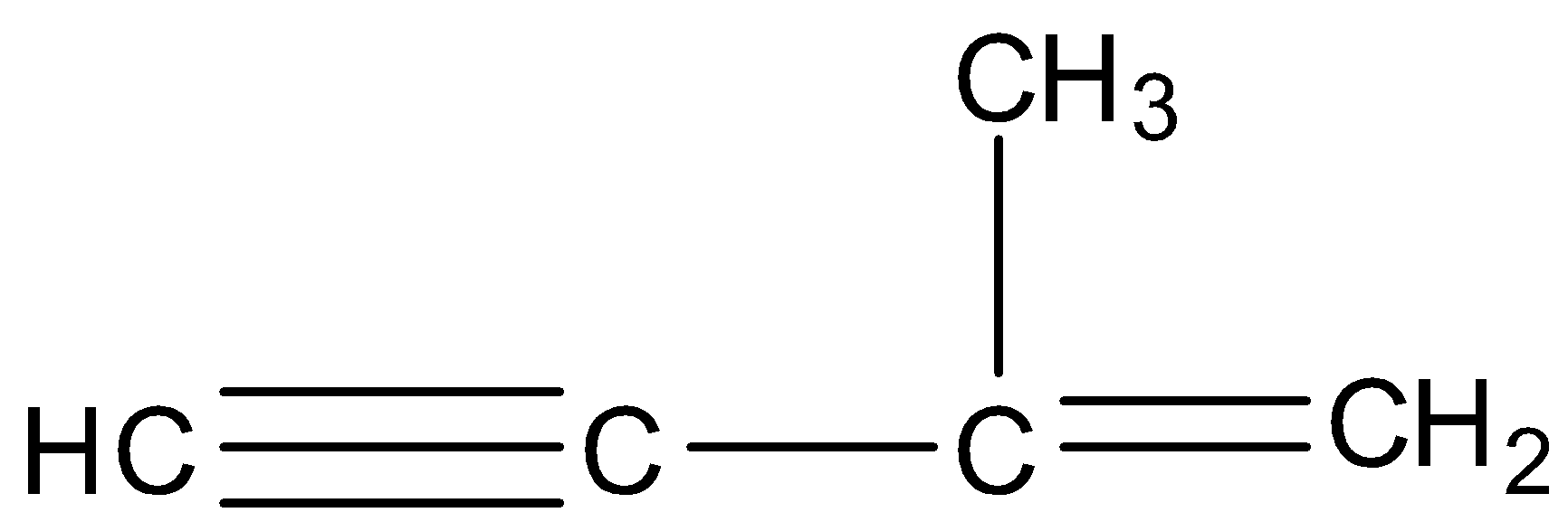
D.
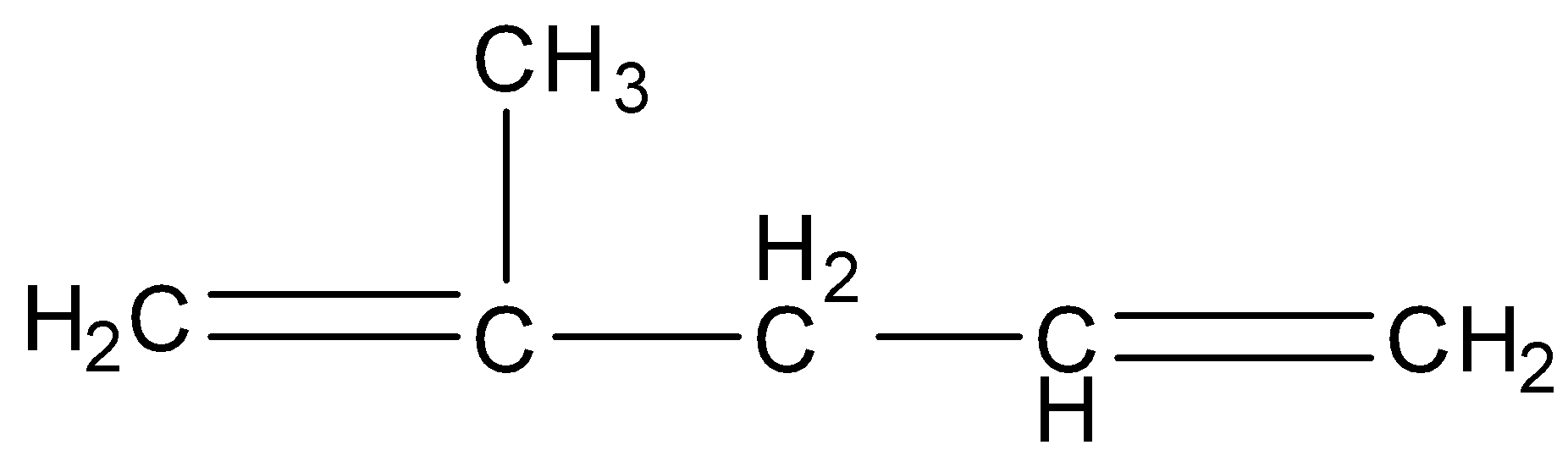


Answer
585.9k+ views
Hint: Isoprene has the chemical formula \[{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{8}}}\]. A structural formula of a compound represents the structure of a particular compound. The prefix “iso” is used when all carbon atoms form a continuous chain except one.
Complete step by step answer:
We know that isoprene is an unsaturated penta-hydrocarbon. Its chemical formula is \[{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{8}}}\] and we can give the structural formula as \[{\text{C}}{{\text{H}}_{\text{2}}} = {\text{C}}\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right) - {\text{CH}} = {\text{C}}{{\text{H}}_{\text{2}}}\].
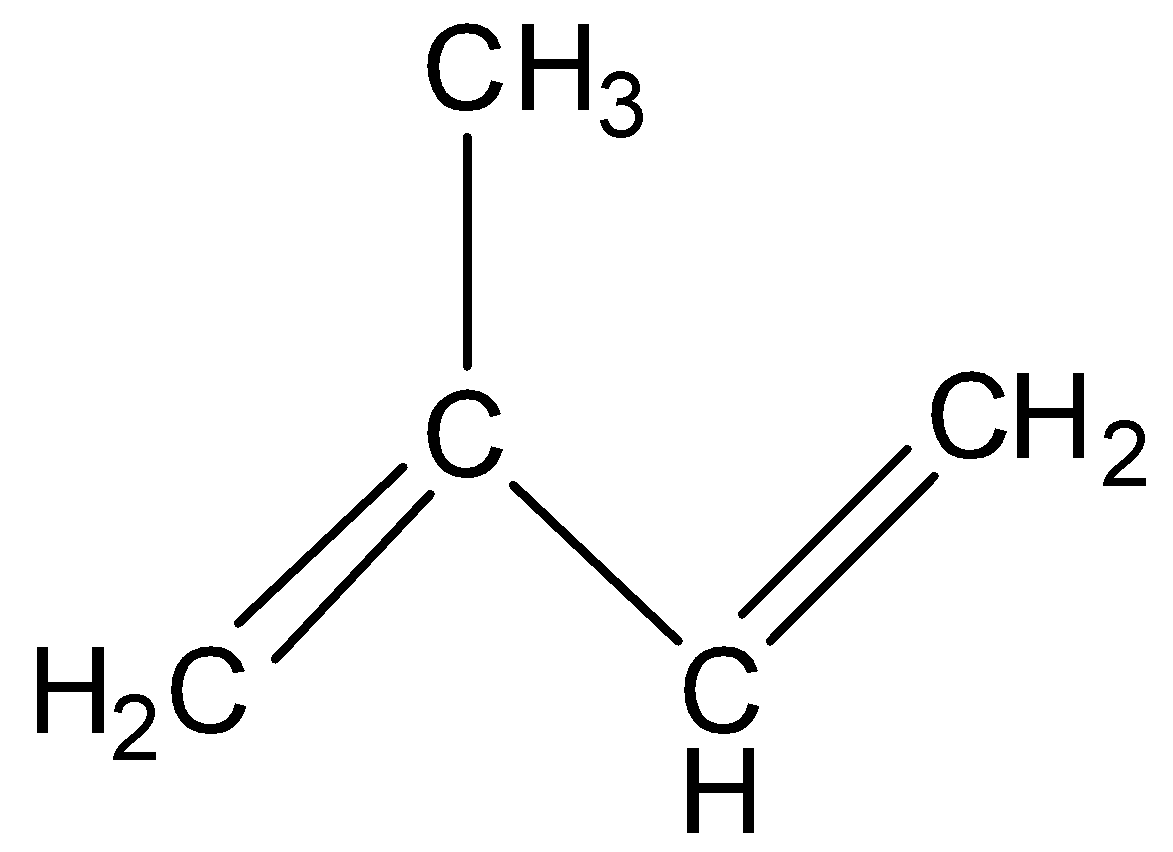
Therefore all of the four given options are incorrect.
Additional information:
Isoprene is produced by many species such as polars, oaks, eucalyptus etc. Isoprene is made through the MEP pathway (methyl-erythritol 4-phosphate pathway) also called as non-mevalonate pathway in the chloroplast present in plants. Isoprene protects the plants against moderate heat stress. It also protects plants against the large fluctuations in the leaf temperature. Isoprene is incorporated and it helps in stabilizing the cell membranes in response to the heat stress. Isoprene is the most abundantly found hydrocarbon in the breath of humans. The production rate of isoprene in the human body is estimated to be \[0.15\,{\text{mu mol/kg}}{\text{.h}}\] which is equivalent to approximately \[17\;{\text{mg/day}}\] for one person who has a weight of \[{\text{70}}\,{\text{kg}}\] Isoprene is commonly found in the low concentration in many of the foods. Many species of soil as well as marine bacteria are capable of degrading available isoprene and that can be used as a fuel source.
Note:
Isoprene can also be named as 2-methyl-1,3-butadiene. In the thermal cracking of oil, isoprene is found as a by-product, and also in the production of ethylene, found as a side product.
Complete step by step answer:
We know that isoprene is an unsaturated penta-hydrocarbon. Its chemical formula is \[{{\text{C}}_{\text{5}}}{{\text{H}}_{\text{8}}}\] and we can give the structural formula as \[{\text{C}}{{\text{H}}_{\text{2}}} = {\text{C}}\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right) - {\text{CH}} = {\text{C}}{{\text{H}}_{\text{2}}}\].

Therefore all of the four given options are incorrect.
Additional information:
Isoprene is produced by many species such as polars, oaks, eucalyptus etc. Isoprene is made through the MEP pathway (methyl-erythritol 4-phosphate pathway) also called as non-mevalonate pathway in the chloroplast present in plants. Isoprene protects the plants against moderate heat stress. It also protects plants against the large fluctuations in the leaf temperature. Isoprene is incorporated and it helps in stabilizing the cell membranes in response to the heat stress. Isoprene is the most abundantly found hydrocarbon in the breath of humans. The production rate of isoprene in the human body is estimated to be \[0.15\,{\text{mu mol/kg}}{\text{.h}}\] which is equivalent to approximately \[17\;{\text{mg/day}}\] for one person who has a weight of \[{\text{70}}\,{\text{kg}}\] Isoprene is commonly found in the low concentration in many of the foods. Many species of soil as well as marine bacteria are capable of degrading available isoprene and that can be used as a fuel source.
Note:
Isoprene can also be named as 2-methyl-1,3-butadiene. In the thermal cracking of oil, isoprene is found as a by-product, and also in the production of ethylene, found as a side product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




