
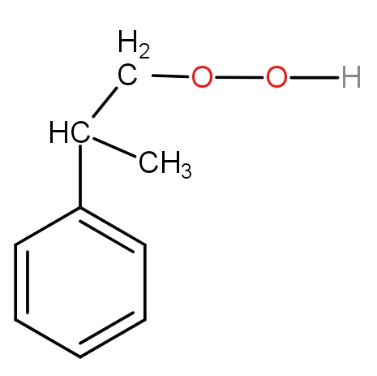
The structure of intermediate A in the following reaction is :
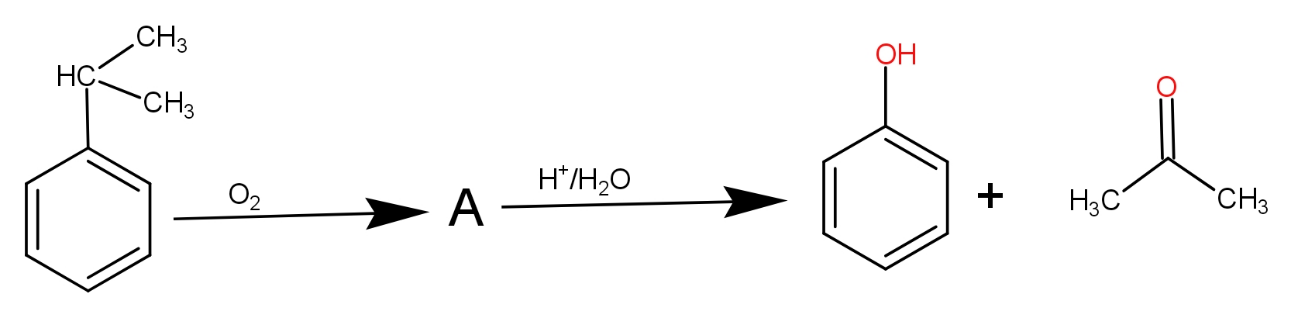
a.)
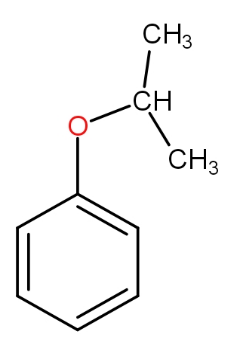
b.)
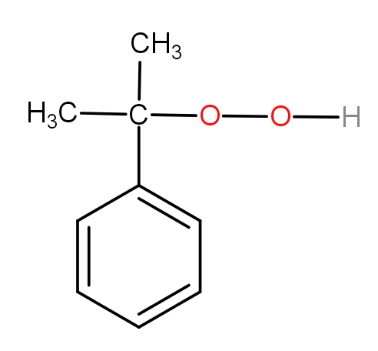
c.)
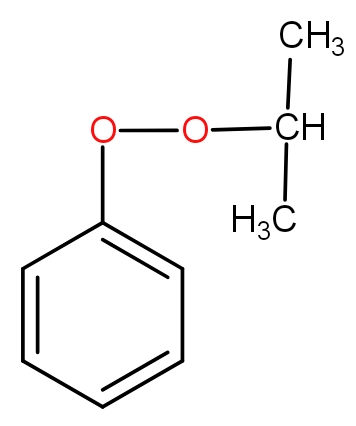
d.)





Answer
577.8k+ views
Hint:. The reaction above is of cumene in which the dioxygen molecule gets incorporated in the molecule by forming bonds with tertiary carbon present. So, the intermediate involves an intermediate which has a carbon-oxygen bond at tertiary carbon. The intermediate has molecular mass 152.19 g/mol.
Complete step by step answer:
The substrate given to us is cumene. The first step involves reagent oxygen molecule which normally gets incorporated in the molecule and thus do oxidation of the molecule. Cumene reacts with oxygen molecules and leads to formation of cumene hydroperoxide which is A. This cumene hydroperoxide on hydrolysis gives phenol and acetone. The reaction is as -
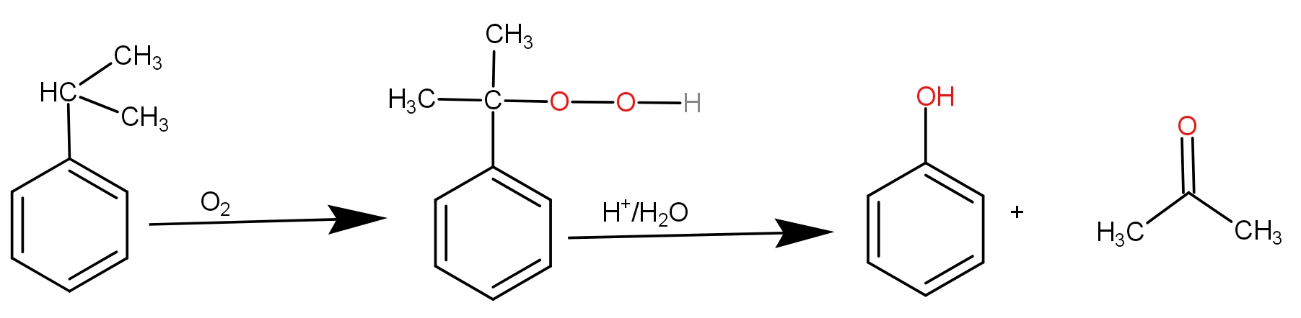
So, the correct answer is “Option B”.
Additional Information:
Cumene has another name isopropyl benzene is a flammable colourless liquid. It is a constituent of crude oil and refined fuel. Most of the cumene is converted to cumene hydroperoxide and this cumene hydroperoxide acts as catalyst for the oxidation of many compounds.
The synthesis of cumene is by Friedel Crafts alkylation reaction of benzene in which the benzene gets substituted by propylene. This cumene if given long exposure to air gives cumene peroxides.
Note: Cumene hydroperoxide is used as an oxidizing agent. It is even involved as an organic peroxide for manufacturing of propylene oxide by oxidation of propylene. It is colourless liquid with sharp and irritating odour. It is used in production of acetone and phenol as a polymerization catalyst.
Complete step by step answer:
The substrate given to us is cumene. The first step involves reagent oxygen molecule which normally gets incorporated in the molecule and thus do oxidation of the molecule. Cumene reacts with oxygen molecules and leads to formation of cumene hydroperoxide which is A. This cumene hydroperoxide on hydrolysis gives phenol and acetone. The reaction is as -

So, the correct answer is “Option B”.
Additional Information:
Cumene has another name isopropyl benzene is a flammable colourless liquid. It is a constituent of crude oil and refined fuel. Most of the cumene is converted to cumene hydroperoxide and this cumene hydroperoxide acts as catalyst for the oxidation of many compounds.
The synthesis of cumene is by Friedel Crafts alkylation reaction of benzene in which the benzene gets substituted by propylene. This cumene if given long exposure to air gives cumene peroxides.
Note: Cumene hydroperoxide is used as an oxidizing agent. It is even involved as an organic peroxide for manufacturing of propylene oxide by oxidation of propylene. It is colourless liquid with sharp and irritating odour. It is used in production of acetone and phenol as a polymerization catalyst.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




