
The structure of bicyclo $[1.1.0]$ butane is
A
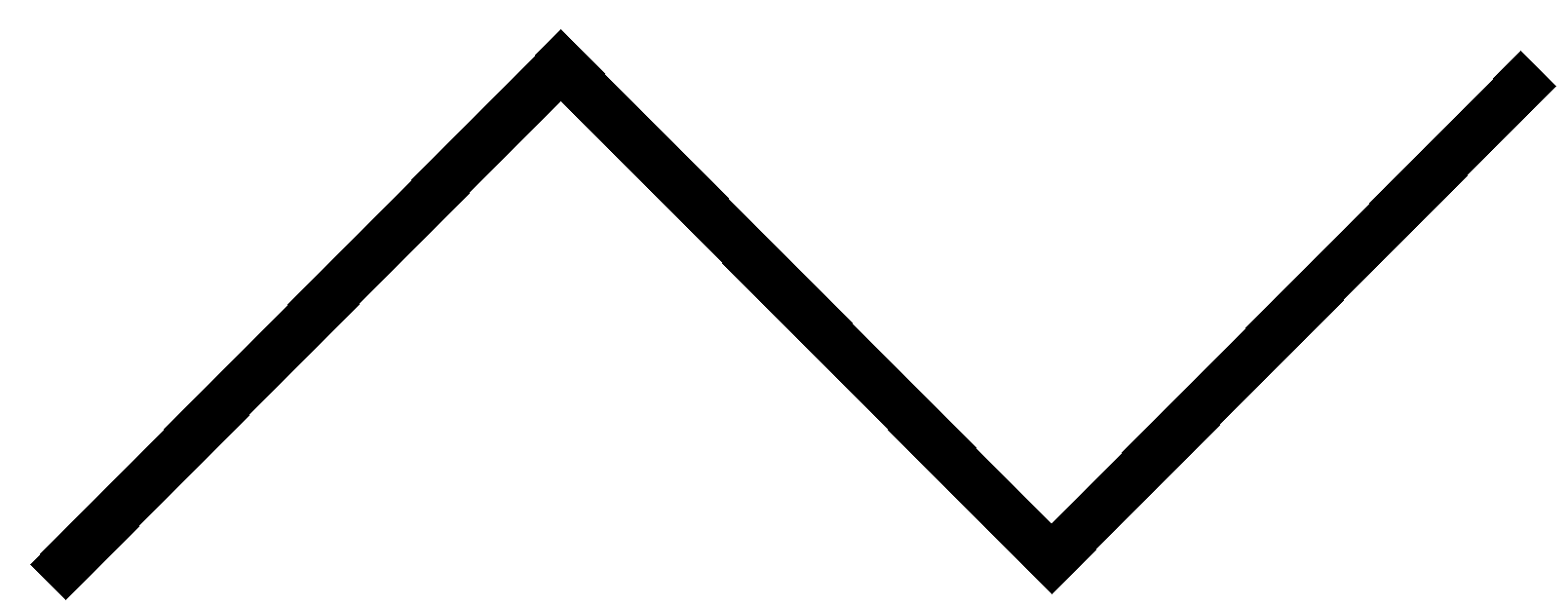
B

C
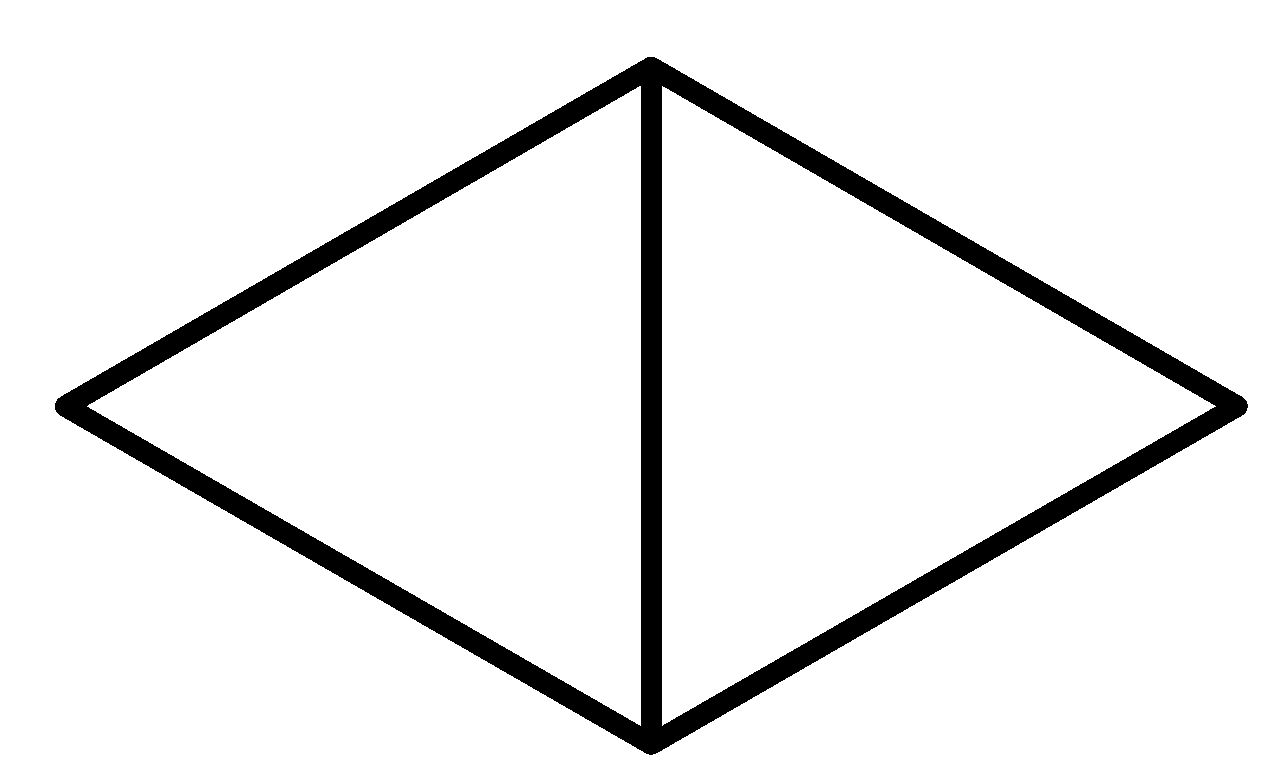
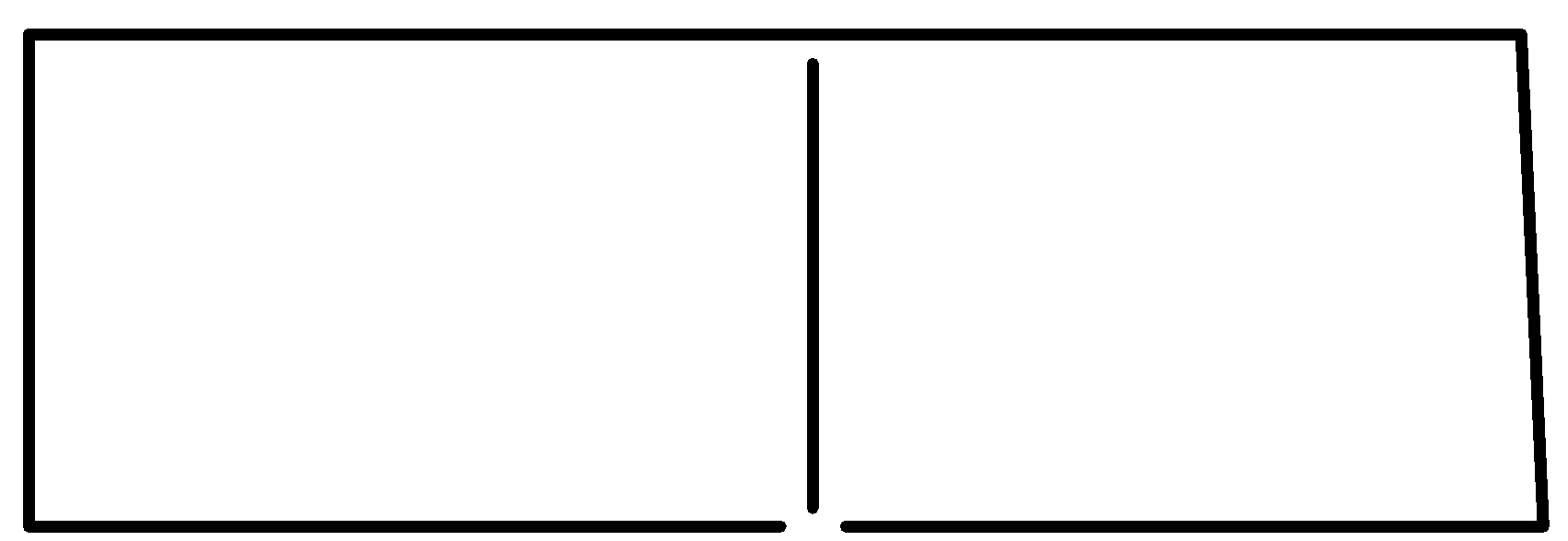
D




Answer
576.3k+ views
Hint: We know that bicycle $[1.1.0]$ butane is a cyclic compound with formula ${C_4}{H_6}$ .
Bicyclo means there are 2 cyclic rings. Since the name is butane, there would be 4 carbon atoms. In a bicyclic compound IUPAC name, the use of the name of alkane corresponds to the number of carbons present in the structure or in the parent chain.
Complete step by step answer:
We know that the alkane given in the question is butane. Therefore, the parent chain is butane. It means that it has 4 carbon atoms present in the structure.
According to the rule of naming bicyclic compounds, the carbon atom which is common to both the rings are called bridgeheads. The bond or atom that connects the bridgeheads is a bridge.
After that the bridge lengths are \[1,1,0\] which means two carbon atoms are common.
Then we see that each ring has one carbon atom which is not common.
Also, we can say that in each ring, there are three Carbon atom out of which 2 are common to both the rings and 1 is not common [1.1.0]
So considering all the given rules and situations the structure which fits in these conditions is option C.
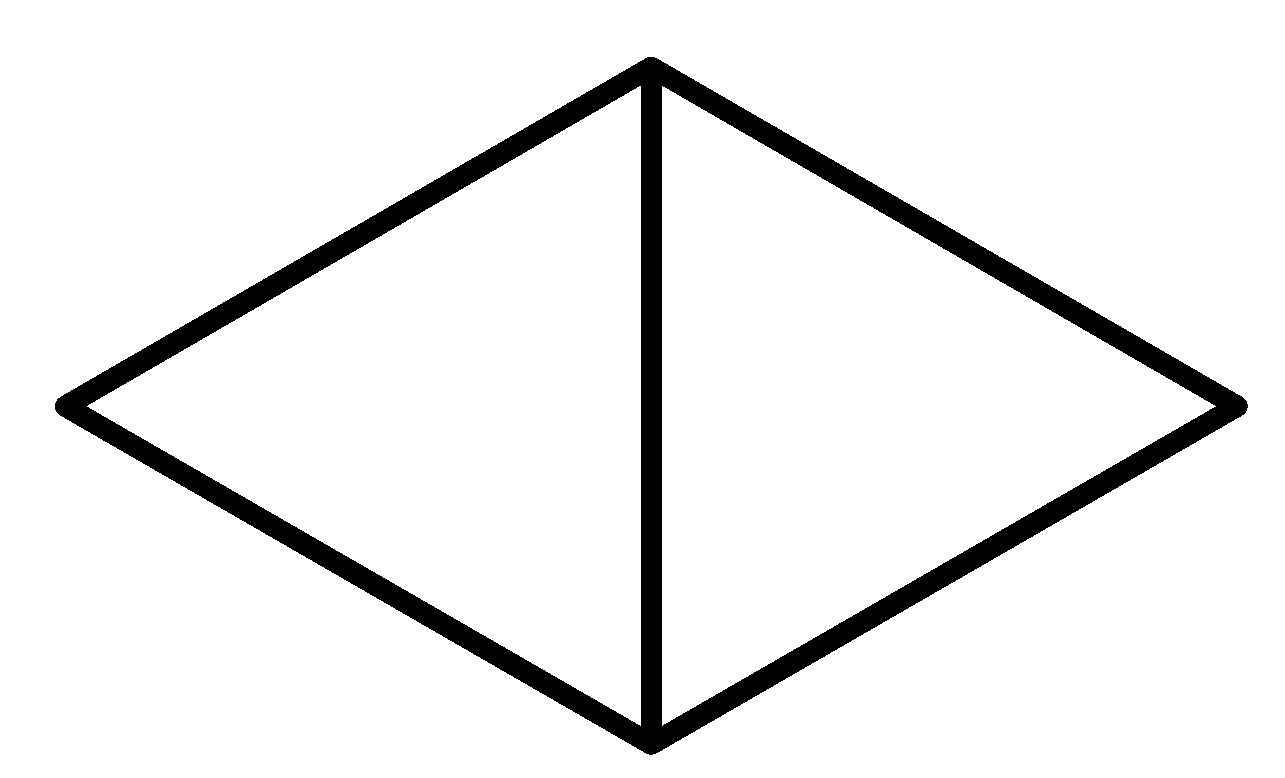
So, the correct answer is Option C.
Note: It is a bicyclic molecule consisting of two cis fused cyclopropane rings. It is a colorless gas and is one of the most strained compounds present on earth. Option A is a normal butane. B is a cyclo butane and it is not bicyclic. In option D, the total number of Carbons in the parent chain is 6 which is against the rule of naming bicyclic compounds.
Bicyclo means there are 2 cyclic rings. Since the name is butane, there would be 4 carbon atoms. In a bicyclic compound IUPAC name, the use of the name of alkane corresponds to the number of carbons present in the structure or in the parent chain.
Complete step by step answer:
We know that the alkane given in the question is butane. Therefore, the parent chain is butane. It means that it has 4 carbon atoms present in the structure.
According to the rule of naming bicyclic compounds, the carbon atom which is common to both the rings are called bridgeheads. The bond or atom that connects the bridgeheads is a bridge.
After that the bridge lengths are \[1,1,0\] which means two carbon atoms are common.
Then we see that each ring has one carbon atom which is not common.
Also, we can say that in each ring, there are three Carbon atom out of which 2 are common to both the rings and 1 is not common [1.1.0]
So considering all the given rules and situations the structure which fits in these conditions is option C.

So, the correct answer is Option C.
Note: It is a bicyclic molecule consisting of two cis fused cyclopropane rings. It is a colorless gas and is one of the most strained compounds present on earth. Option A is a normal butane. B is a cyclo butane and it is not bicyclic. In option D, the total number of Carbons in the parent chain is 6 which is against the rule of naming bicyclic compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




