
The structure of Bakelite is….
A).
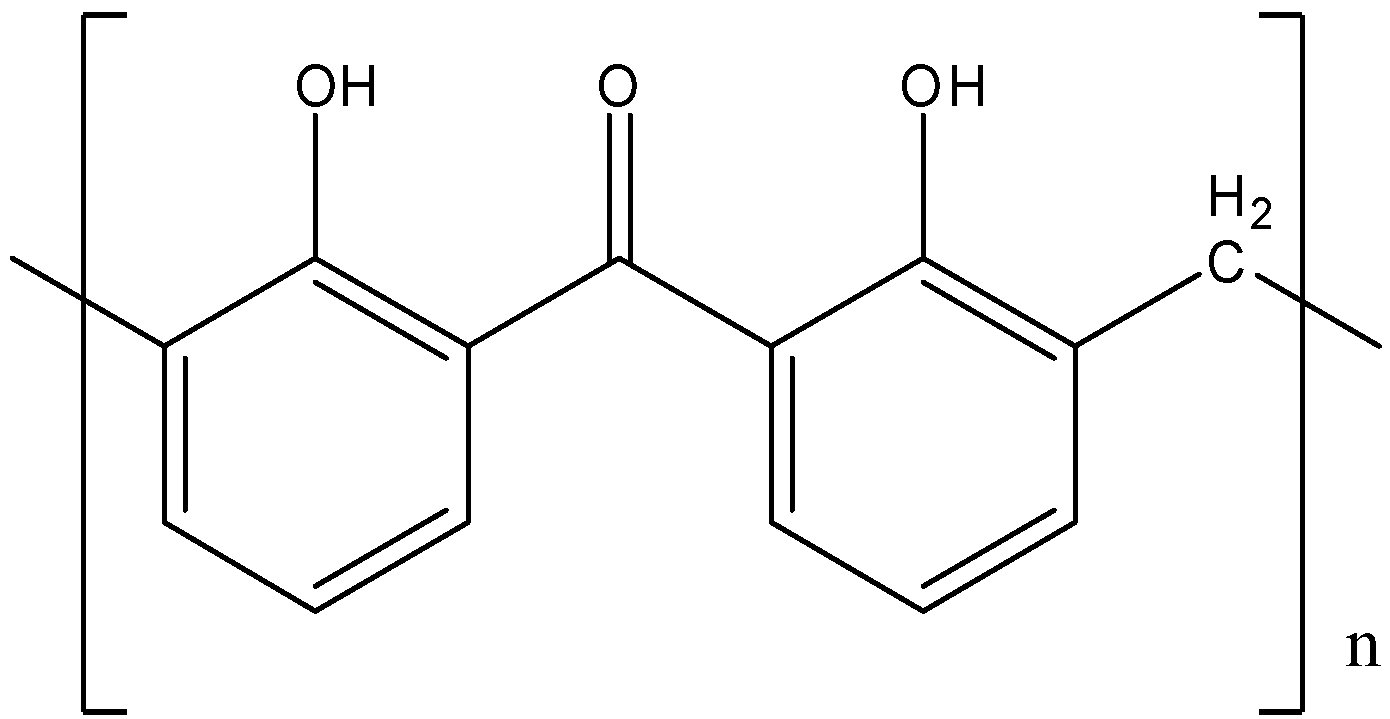
B).
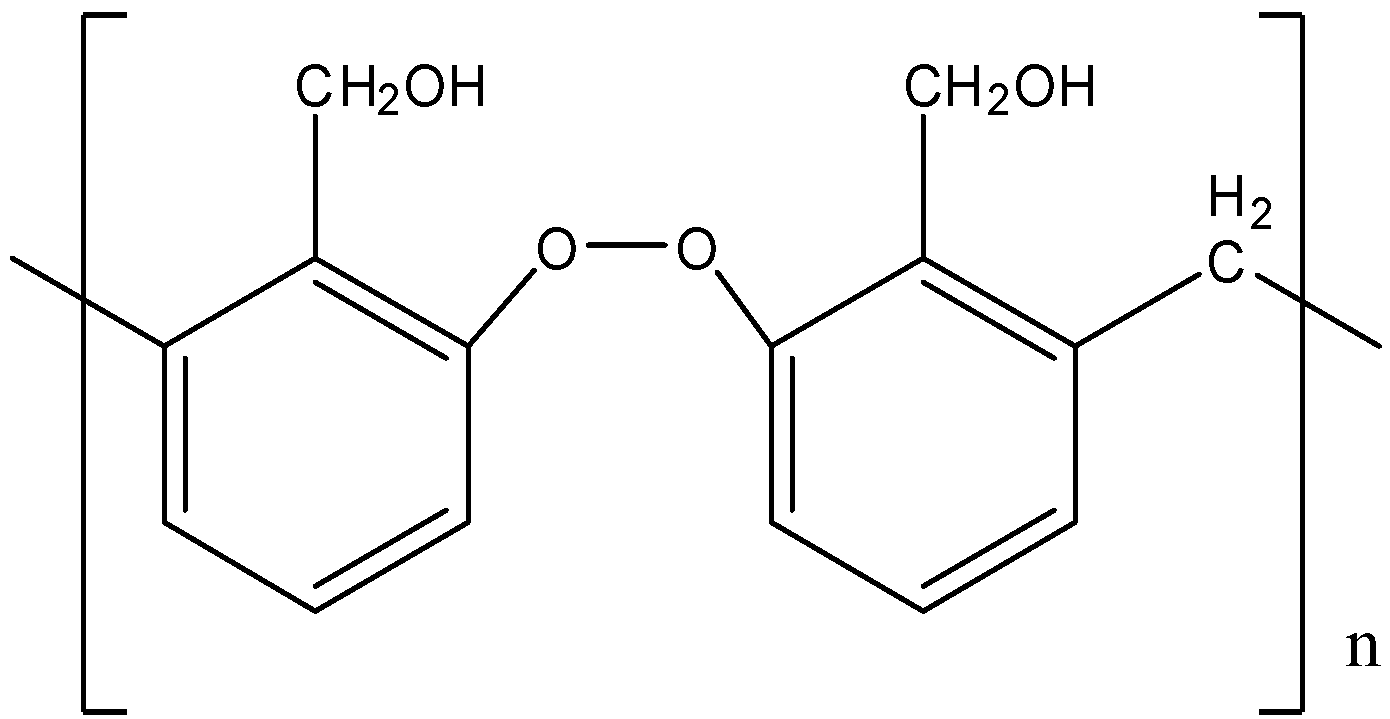
C).
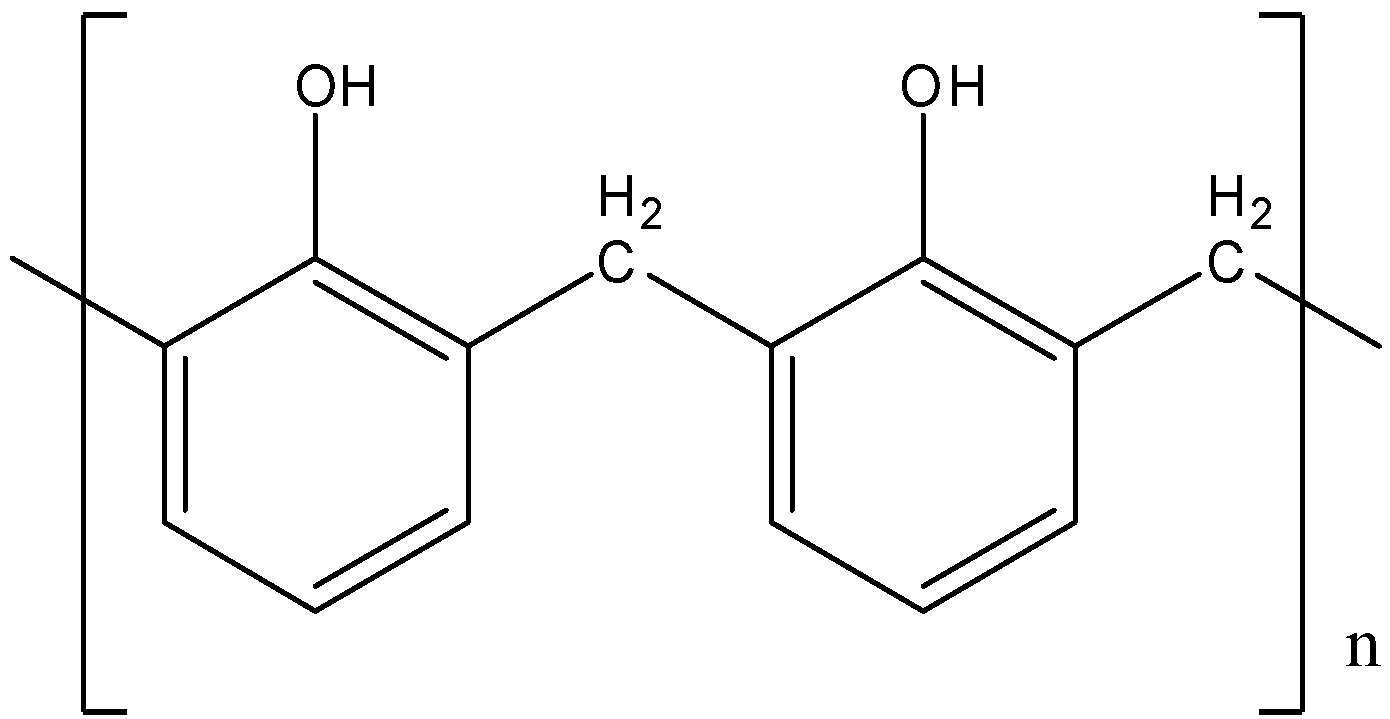
D).
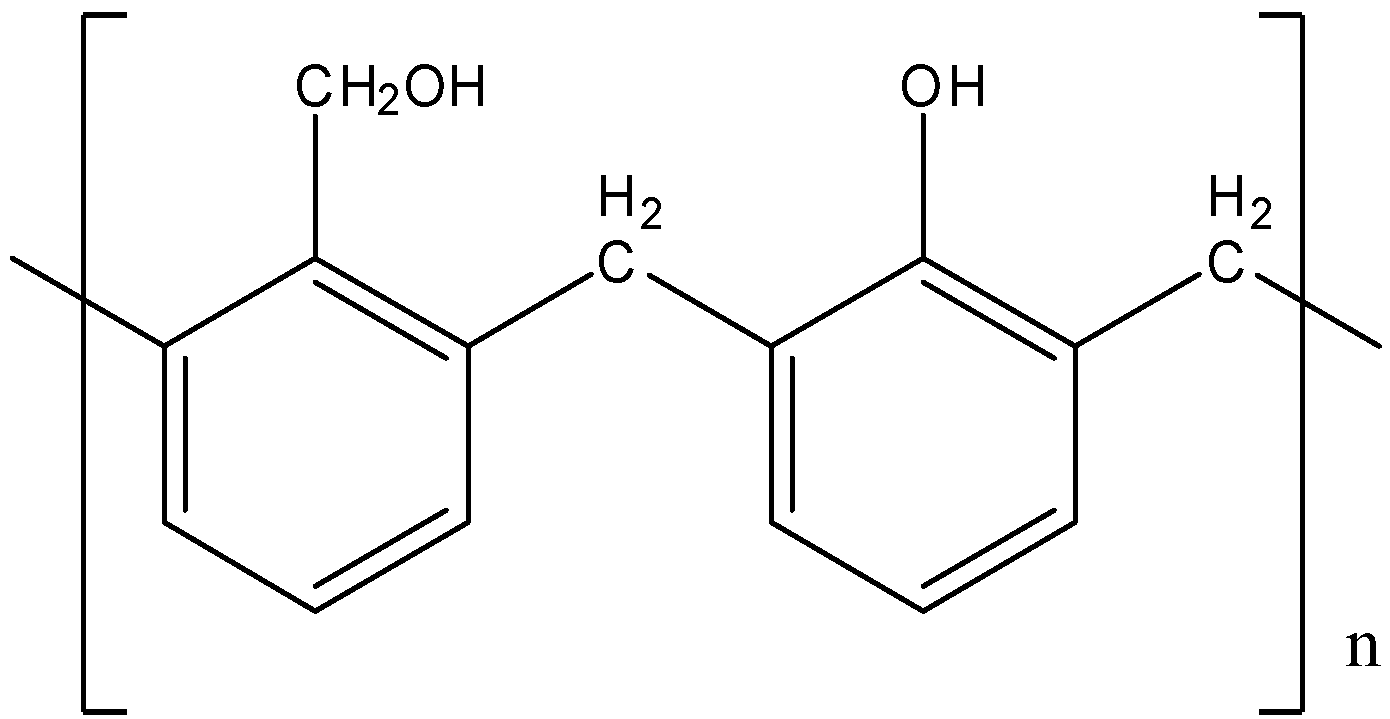




Answer
594.6k+ views
Hint: Bakelite is a type of a co-polymer. It has a compound as a monomer that bears a carbonyl group. Other monomers do not bear any carbonyl group. So, basically it is made up by polymerization of two monomers.
Complete answer:
- Bakelite is also called Phenol-Formaldehyde resin. That means that it is made up of two monomers namely Phenol and Formaldehyde.
- In the synthesis of Bakelite, electrophilic substitution of Formaldehyde carbonyl carbon occurs on Phenol rings. Later on, it loses –OH group and becomes alkene. That reacts with other Phenol molecules and polymerization occurs.
- Bakelite does not have any free carbonyl group in its structure, so option (A) is incorrect.
- It also does not have any type of -O-O- linkage. So, option (B) is also incorrect.
- There is no \[ - C{H_2}OH\] group on benzene rings in the structure of Bakelite.
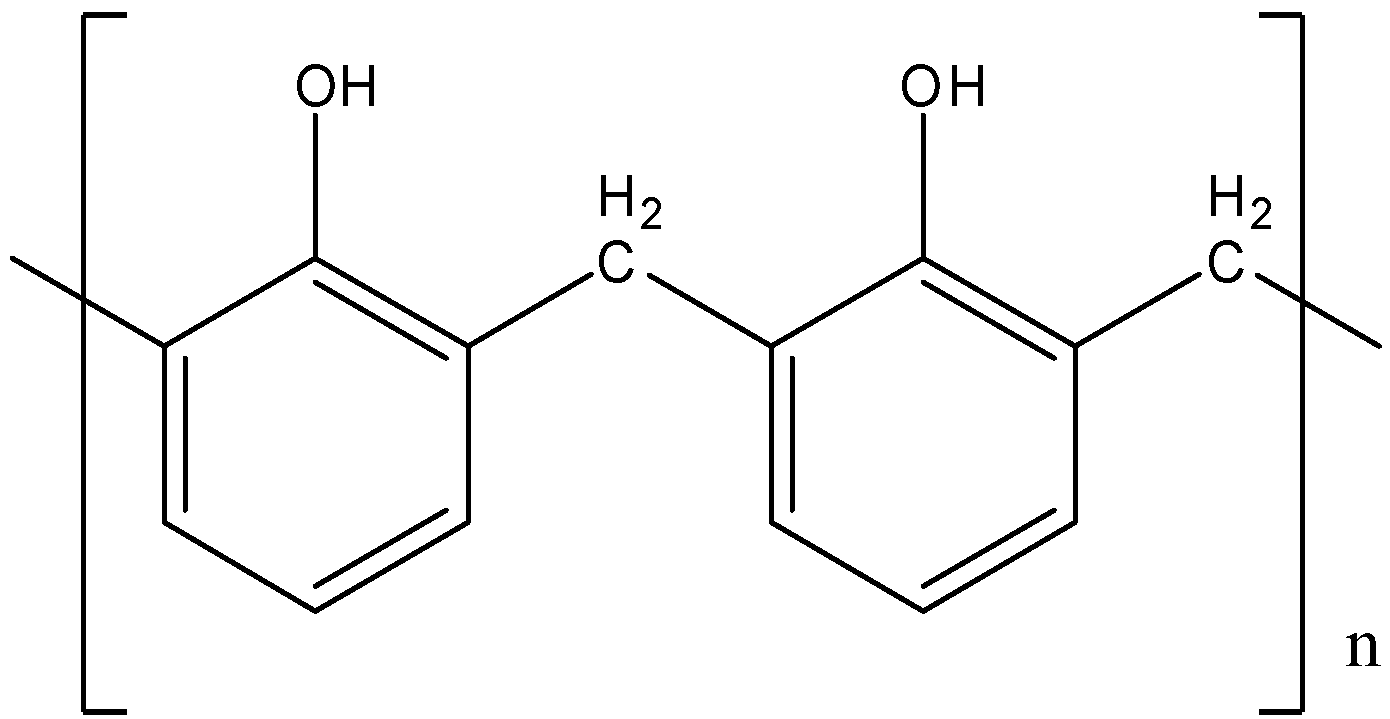
- So, correct answer is option (C)
Additional Information:
- Bakelite is formed by a condensation reaction of Phenol and Formaldehyde. So, Bakelite is a product of condensation polymerization.
- It is a thermosetting type of a polymer. That means upon heating, Bakelite sets to a new more dense material and this process is irreversible.
- Bakelite can be molded quickly and it has heat and scratch resistant properties. It is an insulator of electricity. So, it is widely used in day-to-day life.
Note: As Bakelite has formaldehyde, do not choose a structure that has a carbonyl group as a correct answer as it undergoes condensation and gets removed. In Bakelite, remember that phenolic –OH groups do not form any bond with any molecule, so it remains intact in the structure.
Complete answer:
- Bakelite is also called Phenol-Formaldehyde resin. That means that it is made up of two monomers namely Phenol and Formaldehyde.
- In the synthesis of Bakelite, electrophilic substitution of Formaldehyde carbonyl carbon occurs on Phenol rings. Later on, it loses –OH group and becomes alkene. That reacts with other Phenol molecules and polymerization occurs.
- Bakelite does not have any free carbonyl group in its structure, so option (A) is incorrect.
- It also does not have any type of -O-O- linkage. So, option (B) is also incorrect.
- There is no \[ - C{H_2}OH\] group on benzene rings in the structure of Bakelite.

- So, correct answer is option (C)
Additional Information:
- Bakelite is formed by a condensation reaction of Phenol and Formaldehyde. So, Bakelite is a product of condensation polymerization.
- It is a thermosetting type of a polymer. That means upon heating, Bakelite sets to a new more dense material and this process is irreversible.
- Bakelite can be molded quickly and it has heat and scratch resistant properties. It is an insulator of electricity. So, it is widely used in day-to-day life.
Note: As Bakelite has formaldehyde, do not choose a structure that has a carbonyl group as a correct answer as it undergoes condensation and gets removed. In Bakelite, remember that phenolic –OH groups do not form any bond with any molecule, so it remains intact in the structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




