
The structure of allylene is:
$C{H_3}CH = C{H_2}$
$C{H_3}CH = CHC{H_3}$
$C{H_3}C{H_2}CH = C{H_2}$
$CH3C \equiv CH$
Answer
586.2k+ views
Hint: The chemical formula for allylene is ${C_3}{H_4}$. It is also known as methyl-acetylene, propine or propyne. The structure of the above compound is written according to the IUPAC rule.
Complete step by step answer:
According to IUPAC, there are various rules to write the structure of an organic compound.
According to the rule, first we select the longest carbon chain i.e. parent chain from the word root of the IUPAC name given for an organic compound. So here the word root is the prop that means there are three carbon atoms connected in a parent chain.
$C - C - C$
In the second step we number the chain in any direction. It shown below,
$\mathop C\limits^3 - \mathop C\limits^2 - \mathop C\limits^1 $
In the third step, we identify the primary suffix i.e. ane, ene, yne from the name of the compound. As we know here, yne is used as the primary suffix so we can say there is a triple bond.
Now we will identify the position of the triple bond. Since, in IUPAC name there is no mention of the position of triple bond that means it should be in the first position.
$\mathop C\limits^3 - \mathop C\limits^2 \equiv \mathop C\limits^1 $
And at last, we attach the required number of the hydrogen atom to satisfy the valency of carbon.
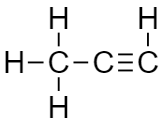
As it contains a triple bond that means it is an unsaturated compound that is unstable and highly reactive.
So, the correct answer is Option D .
Note:
Allylene appears as colourless liquefied gas with a sweet odour. It is insoluble in water but soluble in ethanol, chloroform and benzene i.e. organic solvents. It is moderately toxic by inhalation. According to European space companies research allylene would be highly advantageous as a rocket fuel for craft intended for low earth orbital operations.
Complete step by step answer:
According to IUPAC, there are various rules to write the structure of an organic compound.
According to the rule, first we select the longest carbon chain i.e. parent chain from the word root of the IUPAC name given for an organic compound. So here the word root is the prop that means there are three carbon atoms connected in a parent chain.
$C - C - C$
In the second step we number the chain in any direction. It shown below,
$\mathop C\limits^3 - \mathop C\limits^2 - \mathop C\limits^1 $
In the third step, we identify the primary suffix i.e. ane, ene, yne from the name of the compound. As we know here, yne is used as the primary suffix so we can say there is a triple bond.
Now we will identify the position of the triple bond. Since, in IUPAC name there is no mention of the position of triple bond that means it should be in the first position.
$\mathop C\limits^3 - \mathop C\limits^2 \equiv \mathop C\limits^1 $
And at last, we attach the required number of the hydrogen atom to satisfy the valency of carbon.

As it contains a triple bond that means it is an unsaturated compound that is unstable and highly reactive.
So, the correct answer is Option D .
Note:
Allylene appears as colourless liquefied gas with a sweet odour. It is insoluble in water but soluble in ethanol, chloroform and benzene i.e. organic solvents. It is moderately toxic by inhalation. According to European space companies research allylene would be highly advantageous as a rocket fuel for craft intended for low earth orbital operations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




