
The species which can best serve as an initiator for the cationic polymerization is:
A. \[LiAl{H_4}\]
B. \[HN{O_3}\]
C. \[AlC{l_3}\]
D. BaLi
Answer
582.6k+ views
Hint: Initiator in a chemical reaction can be understood as the chemical species that can react with the monomer to form an intermediate compound. This intermediate compound is capable of linking itself with many other similar monomeric molecules, thus resulting in the formation of a polymer. To put this in simpler terms, an initiator is basically that compound that reacts with the monomer to make it capable of forming polymeric molecules.
Complete step by step answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Cationic polymerization can be understood as the chain growth polymerization in which the contributing monomer is made reactive with the help of a cationic initiator. To put it in simpler terms, cationic polymerization can be explained as an ionic polymerization reaction in which the kinetic chain carriers are cations.
Now that we have seen that for a cationic polymerization reaction, we would be requiring a cationic initiator, we can look through the options to find a cationic initiator or basically a Lewis acid. Lewis acids are chemical species that are electrophilic in nature, i.e. they accept lone pairs of electrons.
The molecular structures of the given compounds can be given as:
\[LiAl{H_4}\]
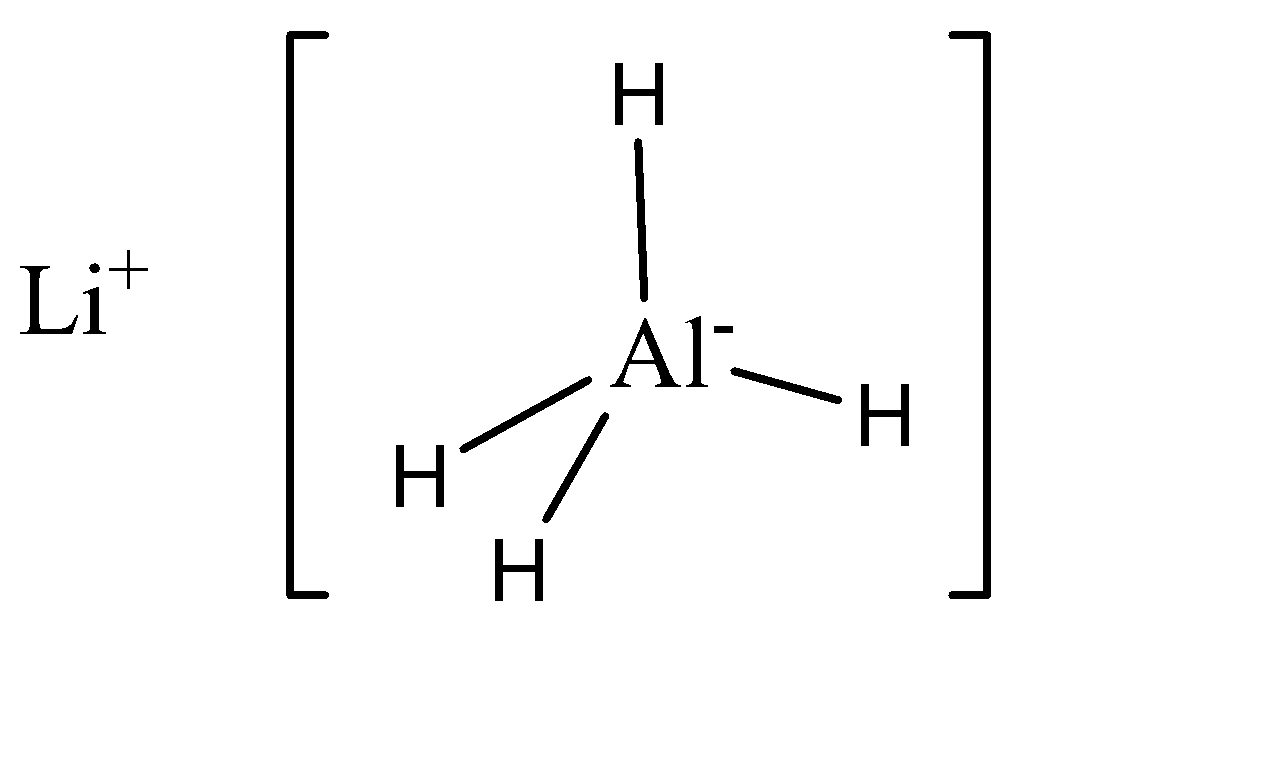
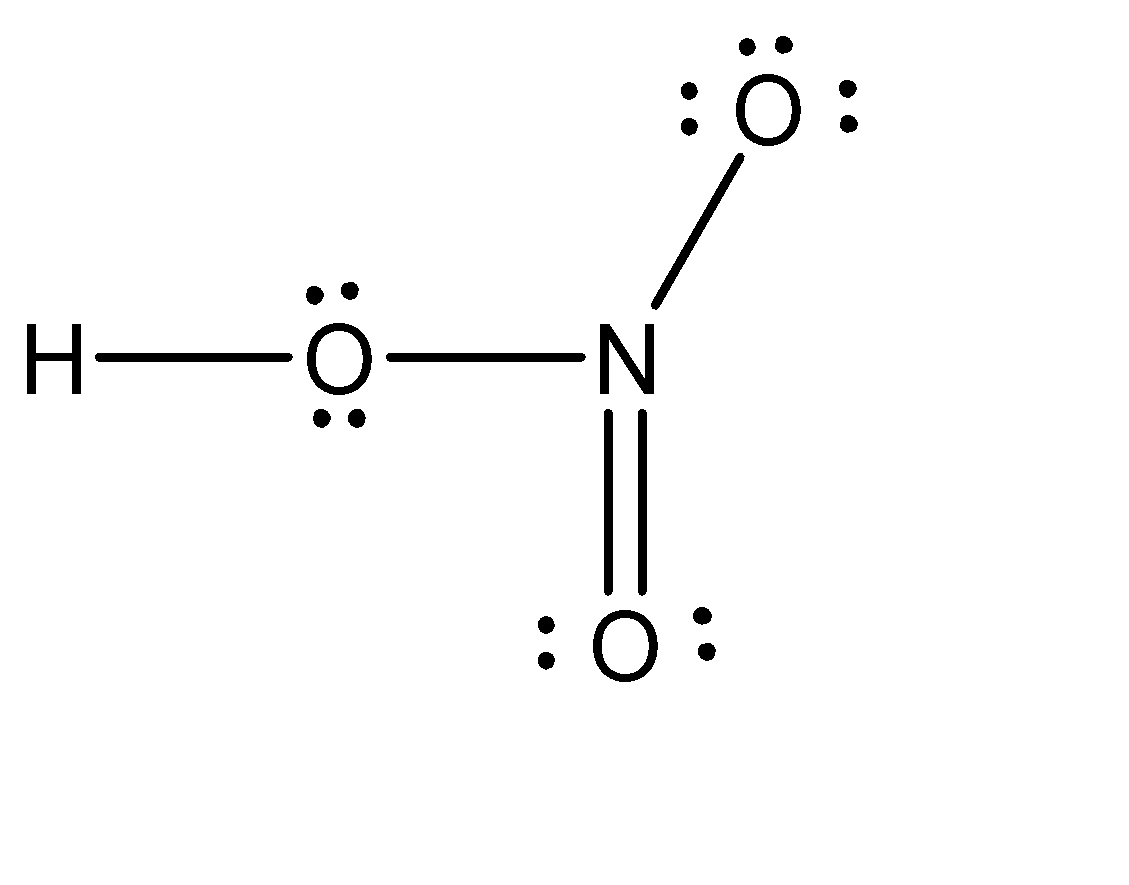
\[HN{O_3}\]
\[AlC{l_3}\]
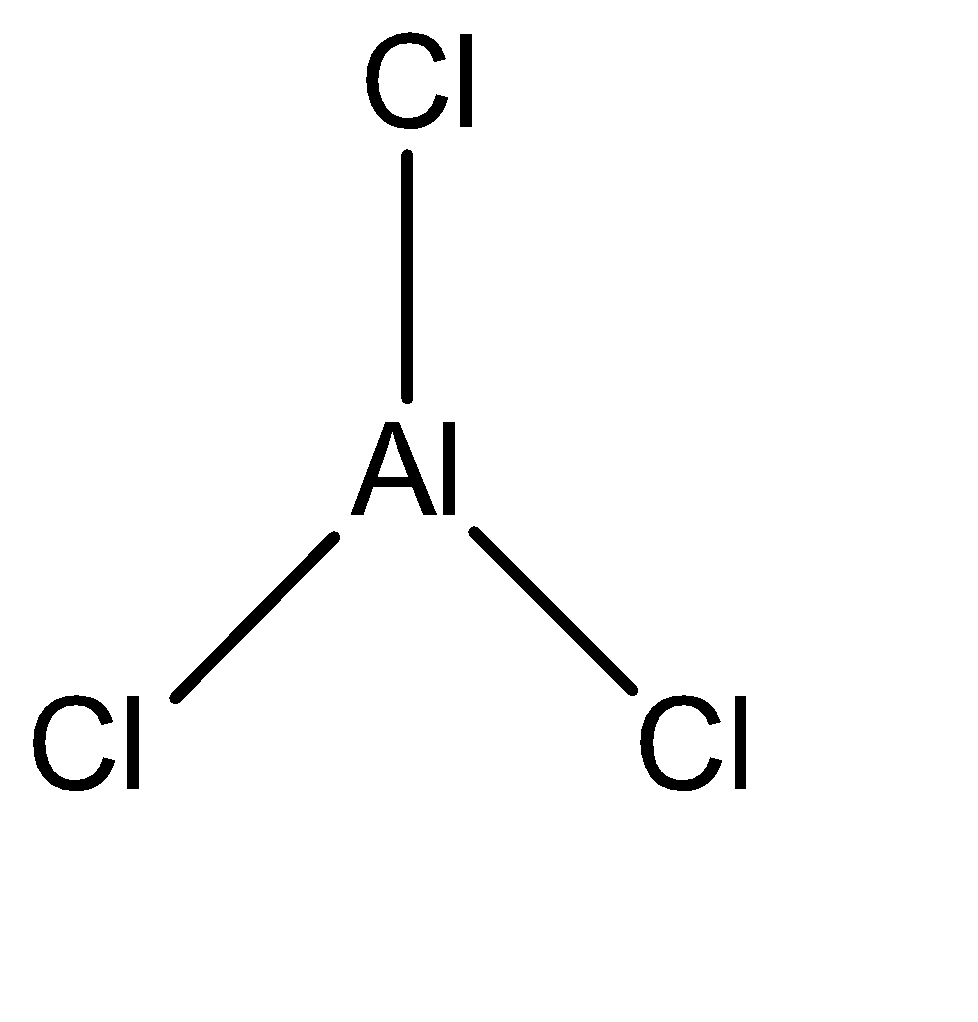
We can observe that Aluminium trichloride is the only Lewis acid in the given set of compounds.
Hence, the species which can best serve as an initiator for the cationic polymerization is \[AlC{l_3}\]
Hence, Option C is the correct option.
Note:
Initiators trigger chemical reactions. They are not true catalysts, as they become an integral part of the product; because of this they are instead considered to be co-reactants.
Complete step by step answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Cationic polymerization can be understood as the chain growth polymerization in which the contributing monomer is made reactive with the help of a cationic initiator. To put it in simpler terms, cationic polymerization can be explained as an ionic polymerization reaction in which the kinetic chain carriers are cations.
Now that we have seen that for a cationic polymerization reaction, we would be requiring a cationic initiator, we can look through the options to find a cationic initiator or basically a Lewis acid. Lewis acids are chemical species that are electrophilic in nature, i.e. they accept lone pairs of electrons.
The molecular structures of the given compounds can be given as:
\[LiAl{H_4}\]

\[HN{O_3}\]

\[AlC{l_3}\]

We can observe that Aluminium trichloride is the only Lewis acid in the given set of compounds.
Hence, the species which can best serve as an initiator for the cationic polymerization is \[AlC{l_3}\]
Hence, Option C is the correct option.
Note:
Initiators trigger chemical reactions. They are not true catalysts, as they become an integral part of the product; because of this they are instead considered to be co-reactants.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




