
The shape of$Xe{O_2}{F_2}$molecule is:
(A)- trigonal bipyramidal
(B)- square planar
(C)- tetrahedral
(D)- see-saw
Answer
569.4k+ views
Hint: The shape of$Xe{O_2}{F_2}$is dependent on the number of atoms joined to the central atom. But, for the shape of the molecule, the number of lone pairs should also be considered and it can be calculated with the help of valence electrons, the number of bonds etc.
Complete step by step answer:
The above question is based on the VSEPR theory and the concept of hybridization which are used to predict the molecular geometries of xenon compounds.
According to the VSEPR theory, the total number of electron pairs (lone pairs + bond pairs) in the valence shell of the central atom (Xenon in this case) is used to predict the shape of the molecule.
To calculate the total number of electron pairs, the following formula is used:
\[\dfrac{{\;\left( {valence{\text{ }}electron{\text{ }}of{\text{ }}central{\text{ }}atom{\text{ }} + {\text{ }}number{\text{ }}of{\text{ }}bonded{\text{ }}atoms} \right)}}{2}\]
In our case, there are 2 fluorine atoms which form a single bond and 2 oxygen atoms which form the double bond. With the above formula, total electron pairs can be calculated as follows:
$\dfrac{{[8 + (2 + 4)]}}{2} = \dfrac{{[8 + (6)]}}{2} = 7$
Hence, there are 7 electron pairs. Since, there are 2 fluorine atoms and 2 oxygen atoms joined to xenon which means that there will be 6 bond pairs of electrons.
Now to calculate number of lone pair, we can use the formula:
Lone pairs = Total number of electron pairs + number of bond pairs
Lone pairs = 8-7 = 1
Hence, there is 1 lone pair in the compound.
Now, depending on the number of covalent bonds by Oxygen and Fluorine with Xenon, the required number of electrons of the 5p-orbital valence shell of Xenon gets unpaired and promoted to the vacant 5-d orbital followed by hybridization.
Considering 7 electron pairs and one oxygen atom has 2 electron pairs, the hybridization will be 5($s{p^3}d$).
So, the hybridization is$s{p^3}d$and it also has one lone pair. Hence, the shape of the given compound will be see-saw as well as trigonal bipyramidal as shown in the figure.
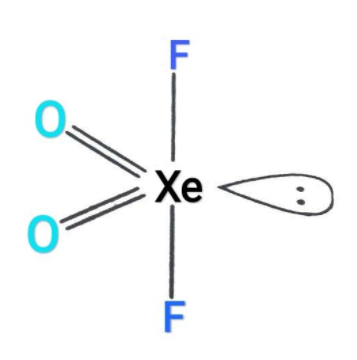
Hence. The correct answers of the question are (A) and (D).
Note: The molecular geometry of the given compound is originally said to be trigonal bipyramidal but due to the presence of lone pair on equatorial position, the actual shape will be see-saw. The repulsion between lone pair and bond pair of electrons will be more. Here, fluorine will be in the axial position and oxygen will be in the equatorial position.
Complete step by step answer:
The above question is based on the VSEPR theory and the concept of hybridization which are used to predict the molecular geometries of xenon compounds.
According to the VSEPR theory, the total number of electron pairs (lone pairs + bond pairs) in the valence shell of the central atom (Xenon in this case) is used to predict the shape of the molecule.
To calculate the total number of electron pairs, the following formula is used:
\[\dfrac{{\;\left( {valence{\text{ }}electron{\text{ }}of{\text{ }}central{\text{ }}atom{\text{ }} + {\text{ }}number{\text{ }}of{\text{ }}bonded{\text{ }}atoms} \right)}}{2}\]
In our case, there are 2 fluorine atoms which form a single bond and 2 oxygen atoms which form the double bond. With the above formula, total electron pairs can be calculated as follows:
$\dfrac{{[8 + (2 + 4)]}}{2} = \dfrac{{[8 + (6)]}}{2} = 7$
Hence, there are 7 electron pairs. Since, there are 2 fluorine atoms and 2 oxygen atoms joined to xenon which means that there will be 6 bond pairs of electrons.
Now to calculate number of lone pair, we can use the formula:
Lone pairs = Total number of electron pairs + number of bond pairs
Lone pairs = 8-7 = 1
Hence, there is 1 lone pair in the compound.
Now, depending on the number of covalent bonds by Oxygen and Fluorine with Xenon, the required number of electrons of the 5p-orbital valence shell of Xenon gets unpaired and promoted to the vacant 5-d orbital followed by hybridization.
Considering 7 electron pairs and one oxygen atom has 2 electron pairs, the hybridization will be 5($s{p^3}d$).
So, the hybridization is$s{p^3}d$and it also has one lone pair. Hence, the shape of the given compound will be see-saw as well as trigonal bipyramidal as shown in the figure.

Hence. The correct answers of the question are (A) and (D).
Note: The molecular geometry of the given compound is originally said to be trigonal bipyramidal but due to the presence of lone pair on equatorial position, the actual shape will be see-saw. The repulsion between lone pair and bond pair of electrons will be more. Here, fluorine will be in the axial position and oxygen will be in the equatorial position.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




