
The shape of sulphate ion is:
A. Hexagonal
B. Square planar
C. Trigonal bipyramidal
D. Tetrahedral
Answer
567.3k+ views
Hint: The sulphate ion is the anion with negative charge. The empirical formula of sulphate ion is $SO_4^{2 - }$ where the central atom is sulphur and it is bonded to two oxygen atoms with double bonds and two oxygen atoms with single bonds. The oxidation state of sulphate ion is -2.
Complete step by step answer:
The sulphate or the sulphate ion is a polyatomic anion which contains different atoms in the anion. The anions are the negatively charged species formed by the loss of electrons. The sulphate ion contains one sulphur atom and four oxygen atoms with -2 oxidation state. The empirical formula of the sulphate ion is $SO_4^{2 - }$. The sulphates are referred to as the salts of sulphuric acid and are prepared from sulphuric acid.
The shape of sulphate ion is shown below.
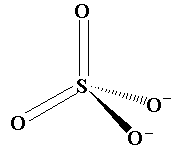
In this structure, the sulphur atom is bonded with two oxygen atoms with double bond and with two oxygen with single bond with -2 oxidation state.
The shape of the sulphate anion is tetrahedral as four bonds are present around the central atom and no lone pair is present on the central atom. The bond angle between the atoms bonded to the central atom is the 109.5 degree.
Thus, the shape of sulphate ions is tetrahedral.
Therefore, the correct option is C.
Note:
The VSEPR theory is applied to find out the geometry and the shape of the molecule depending on the bond pairs and lone pairs present in the structure. The shape of the chemical compound is also based on the hybrid orbital. The molecule or ion with tetrahedral shape has $s{p^3}$ hybridization at the central atom.
Complete step by step answer:
The sulphate or the sulphate ion is a polyatomic anion which contains different atoms in the anion. The anions are the negatively charged species formed by the loss of electrons. The sulphate ion contains one sulphur atom and four oxygen atoms with -2 oxidation state. The empirical formula of the sulphate ion is $SO_4^{2 - }$. The sulphates are referred to as the salts of sulphuric acid and are prepared from sulphuric acid.
The shape of sulphate ion is shown below.

In this structure, the sulphur atom is bonded with two oxygen atoms with double bond and with two oxygen with single bond with -2 oxidation state.
The shape of the sulphate anion is tetrahedral as four bonds are present around the central atom and no lone pair is present on the central atom. The bond angle between the atoms bonded to the central atom is the 109.5 degree.
Thus, the shape of sulphate ions is tetrahedral.
Therefore, the correct option is C.
Note:
The VSEPR theory is applied to find out the geometry and the shape of the molecule depending on the bond pairs and lone pairs present in the structure. The shape of the chemical compound is also based on the hybrid orbital. The molecule or ion with tetrahedral shape has $s{p^3}$ hybridization at the central atom.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




