
The shape of \[{p_x}\] orbital is_____
Answer
471.3k+ views
Hint: Electrons are sub-atomic particles that are present in the atom. These are found outside the nucleus. The region where the probability of finding an electron is maximum is called an orbital. The orbitals are of different types. p-orbital has three degenerate orbitals and the shape is a dumbbell.
Complete step by step answer:
Atoms are tiny particles consisting of sub-atomic particles called neutrons, electrons, and protons. The protons and neutrons are present inside the nucleus where the nucleus is the heavy portion in the center of the atom and the electrons are present outside the nucleus.
The region where the probability of finding an electron is maximum is known as orbitals. The region where the probability of finding an electron is maximum is known as the nodal plane or nodal region.
These orbitals are divided into different types based on the accommodation of electrons in them.
The orbitals are s, p, d, and f. The p-orbital can accommodate six electrons and is sub-divided into three types. These sub-orbitals are \[{p_x},{p_y},\] and \[{p_z}\] orbitals. All these three orbitals have same energy and can be called as degenerate orbitals. The shape of these three orbitals is dumbbell shape.
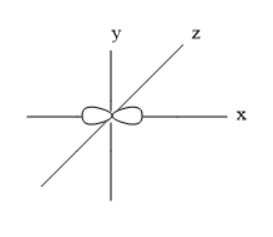
Thus, the shape of \[{p_x}\] orbital Is dumbbell.
Note:
The s-orbital can accommodate two electrons and p-orbital can accommodate six electrons and the degeneracy is three. The d-orbital can accommodate ten electrons and has the degeneracy is five and the f-orbital can accommodate fourteen electrons and has the degeneracy is seven.
Complete step by step answer:
Atoms are tiny particles consisting of sub-atomic particles called neutrons, electrons, and protons. The protons and neutrons are present inside the nucleus where the nucleus is the heavy portion in the center of the atom and the electrons are present outside the nucleus.
The region where the probability of finding an electron is maximum is known as orbitals. The region where the probability of finding an electron is maximum is known as the nodal plane or nodal region.
These orbitals are divided into different types based on the accommodation of electrons in them.
The orbitals are s, p, d, and f. The p-orbital can accommodate six electrons and is sub-divided into three types. These sub-orbitals are \[{p_x},{p_y},\] and \[{p_z}\] orbitals. All these three orbitals have same energy and can be called as degenerate orbitals. The shape of these three orbitals is dumbbell shape.

Thus, the shape of \[{p_x}\] orbital Is dumbbell.
Note:
The s-orbital can accommodate two electrons and p-orbital can accommodate six electrons and the degeneracy is three. The d-orbital can accommodate ten electrons and has the degeneracy is five and the f-orbital can accommodate fourteen electrons and has the degeneracy is seven.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




