
The s-block element used as a catalyst in the manufacture of Buna-S rubber is:
a.) Mg
b.) Ca
c.) Ba
d.) Na
Answer
585k+ views
Hint: To answer the name of the correct catalyst, we should first know about the manufacturing process of Buna-S. The answer of this question is a soft, silvery-white, highly reactive metal.
Complete step by step solution:
Let us know about the manufacturing process of Buna-S rubber. We should know that Buna-S rubber is one of the commonly used synthetic rubbers. It should be noted that it is a random co-polymer formed by the emulsion polymerisation of a mixture of 1:3 butadiene and styrene. So, we can call 1.3-butadiene and styrene as the monomers of Buna-S. We should know that the styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery.
We should note that n Buna-S, Bu stands for butadiene and, Na for sodium and S for styrene. It is vulcanised with sulphur. Let us now observe the reaction:
The above reaction is
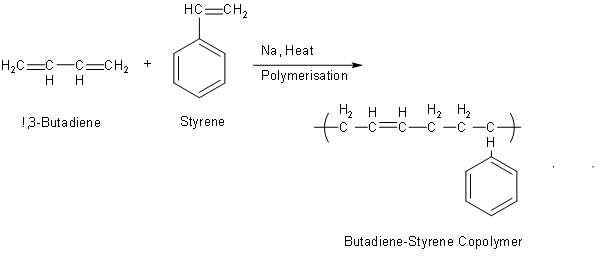
The above reaction is the conversion of 1,3-Butadiene and styrene into Buna S or we can say that it is Butadiene-Styrene Copolymer. As we can see in the reaction, sodium is used for this conversion. So, from the above discussion we can say that the S block element which is used as a catalyst in the manufacture of Buna-S is sodium or Na. The correct answer of this question is option D that is Na.
Note: Now, we will know about the use of Buna-S. We should note that it is used for the manufacture of passenger car tyres and treads, motorcycle and scooter tyres, cycle tyres and tubes. They are also used for the manufacture of conveyor belts, foot-wares, shoe soles, hoses and electrical insulation.
Complete step by step solution:
Let us know about the manufacturing process of Buna-S rubber. We should know that Buna-S rubber is one of the commonly used synthetic rubbers. It should be noted that it is a random co-polymer formed by the emulsion polymerisation of a mixture of 1:3 butadiene and styrene. So, we can call 1.3-butadiene and styrene as the monomers of Buna-S. We should know that the styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery.
We should note that n Buna-S, Bu stands for butadiene and, Na for sodium and S for styrene. It is vulcanised with sulphur. Let us now observe the reaction:
The above reaction is

The above reaction is the conversion of 1,3-Butadiene and styrene into Buna S or we can say that it is Butadiene-Styrene Copolymer. As we can see in the reaction, sodium is used for this conversion. So, from the above discussion we can say that the S block element which is used as a catalyst in the manufacture of Buna-S is sodium or Na. The correct answer of this question is option D that is Na.
Note: Now, we will know about the use of Buna-S. We should note that it is used for the manufacture of passenger car tyres and treads, motorcycle and scooter tyres, cycle tyres and tubes. They are also used for the manufacture of conveyor belts, foot-wares, shoe soles, hoses and electrical insulation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




