
The relative order of esterification of alcohols is:
A. $1 < 2 < 3$
B. $1 > 2 > 3$
C. $1 > 3 > 2$
D. $1 < 3 < 2$
Answer
583.5k+ views
Hint: Ester can be formed by the reaction between acid and alcohol. Reactivity of the alcohols depends upon the \[C - OH\] bond breaking energy. Weaker the \[C - OH\] bond higher will be the reactivity and vice-versa.
Complete step by step answer:
Esterification is a reaction between carboxylic acid and alcohol to produce ester. In this reaction elimination of \[{H_2}O\] molecules takes place. This reaction can be acid catalyzed or base catalyzed. Neutral condition esterification can also be done. The reaction mechanisms are shown below.
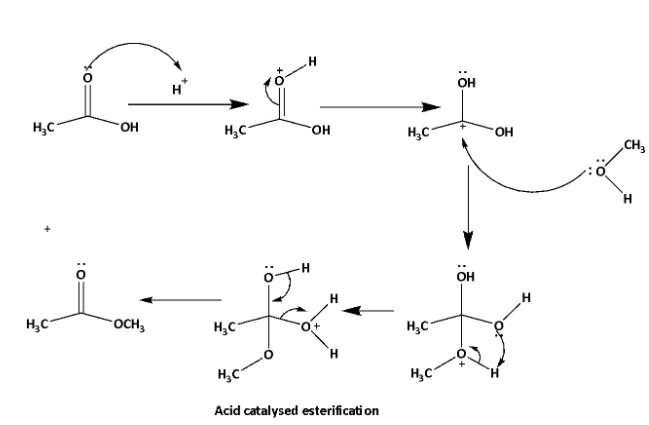
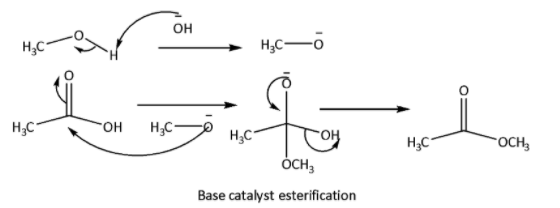
In this reaction reactivity of the alcohols depends upon the \[C - OH\] bond stability. Lower the bond strength higher will be the tendency of esterification reaction. \[C - OH\] bond strength depends upon the inductive effect of the alkyl group. Higher the +I effect of the alkyl group higher will be the bond strength of \[C - OH\] bond, therefore lower will be the esterification tendency.
The +I effect order of the alkyl group is, tertiary group> secondary group > primary group.
Therefore, bond strength order of the \[C - OH\] bond,
tertiary alcohol> secondary alcohol> primary alcohol.
So, the correct option is B.
Note:
Esterification order depends upon the \[C - OH\] bond stability of alcohols. Higher the positive inductive effect of the alkyl group higher the bond strength of \[C - OH\] bond. The order of esterification of alcohols is also because of the increase of steric hindrance from primary to tertiary alcohols. Due to steric hindrance esterification reaction tendency decreases.
Complete step by step answer:
Esterification is a reaction between carboxylic acid and alcohol to produce ester. In this reaction elimination of \[{H_2}O\] molecules takes place. This reaction can be acid catalyzed or base catalyzed. Neutral condition esterification can also be done. The reaction mechanisms are shown below.


In this reaction reactivity of the alcohols depends upon the \[C - OH\] bond stability. Lower the bond strength higher will be the tendency of esterification reaction. \[C - OH\] bond strength depends upon the inductive effect of the alkyl group. Higher the +I effect of the alkyl group higher will be the bond strength of \[C - OH\] bond, therefore lower will be the esterification tendency.
The +I effect order of the alkyl group is, tertiary group> secondary group > primary group.
Therefore, bond strength order of the \[C - OH\] bond,
tertiary alcohol> secondary alcohol> primary alcohol.
So, the correct option is B.
Note:
Esterification order depends upon the \[C - OH\] bond stability of alcohols. Higher the positive inductive effect of the alkyl group higher the bond strength of \[C - OH\] bond. The order of esterification of alcohols is also because of the increase of steric hindrance from primary to tertiary alcohols. Due to steric hindrance esterification reaction tendency decreases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




