
The reagent used for dehalogenation
A. Zn
B. Na
C. Fe
D. Al
Answer
583.2k+ views
Hint: The choice of reagent depends on the capability of the reagent to liberate halogens and also on the energy released during the reaction. The reagent will be good only if the energy released in the reaction is more than the energy needed to break the bond between the halogen and carbon.
Complete step by step solution:
-Halogens are electronegative in nature and they attract the atoms which have less value of electronegativity and make bonds with it to become stable. They are non-metals.
-Carbon is also a non-metal and the bond formed between carbon and the halogens is covalent. This bond is broken down if an electropositive element comes and interacts with the compound of carbon and halogen.
-The halogens will tend to combine with the electropositive elements in order to form ionic bonds between them and this results in dehalogenation. So dehalogenation is the process of removal of halogens in presence of some electropositive reagents.
-As the halogens are to be removed, we need a metal as a reagent for the process. Also, the reaction should be exothermic to be feasible. Exothermic reaction involves release in the energy which makes the reaction products stable.
-Among the options given, all are metals. So the best reagent is checked by the value of ionization energy and the valency also. We need to liberate 1 molecule of halogen and for that, metal with valency 2 should be preferred.
-Zn is the only metal here with valency 2. Also it has very less value of ionization energy and so it results in the exothermic process. This is mandatory as the formation of double bonds is an endothermic process and the overall reaction has to be exothermic.
Therefore the correct option is A. Zn
Reaction for dehalogenation can be shown as
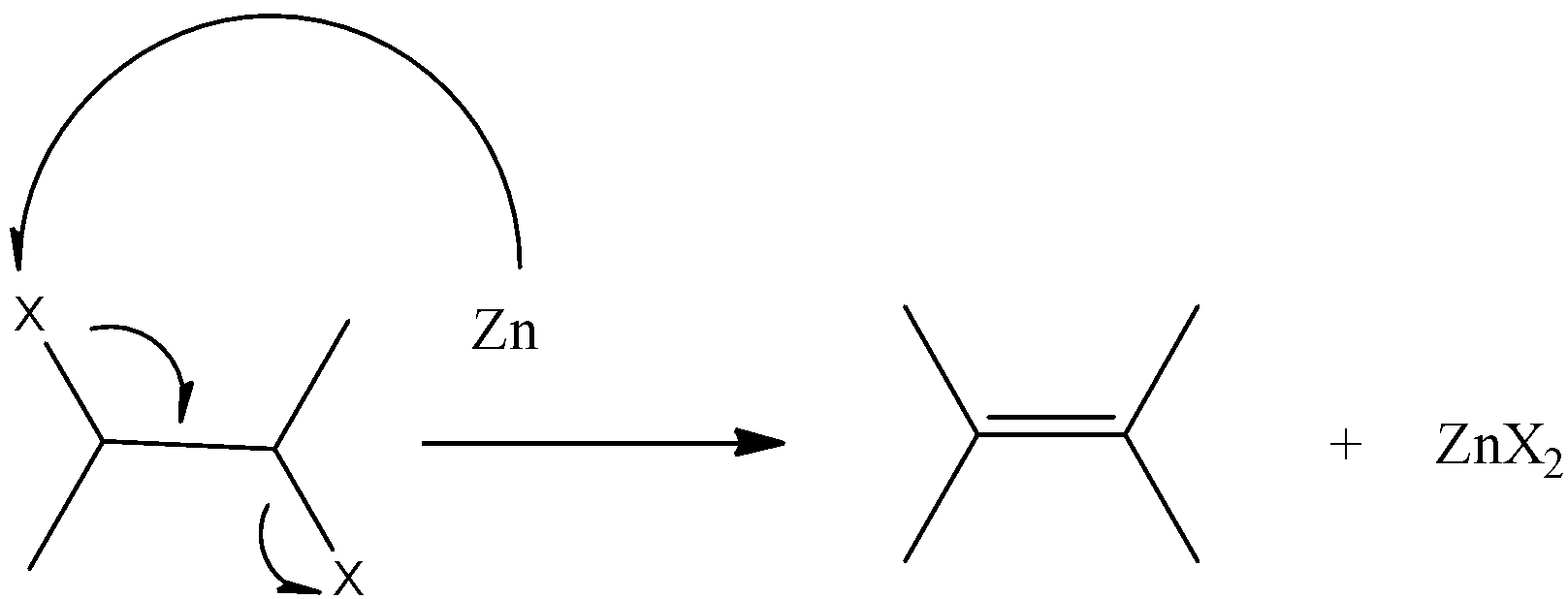
Note: Dehalogenation is different from dehydrohalogenation. In the former, the halogen escapes as a gas and in the form of its molecule with the formula ${{X}_{2}}$ where X represents the halogens. In the latter, HX gas is liberated after the completion of the reaction where X is the single atom of halogen.
Complete step by step solution:
-Halogens are electronegative in nature and they attract the atoms which have less value of electronegativity and make bonds with it to become stable. They are non-metals.
-Carbon is also a non-metal and the bond formed between carbon and the halogens is covalent. This bond is broken down if an electropositive element comes and interacts with the compound of carbon and halogen.
-The halogens will tend to combine with the electropositive elements in order to form ionic bonds between them and this results in dehalogenation. So dehalogenation is the process of removal of halogens in presence of some electropositive reagents.
-As the halogens are to be removed, we need a metal as a reagent for the process. Also, the reaction should be exothermic to be feasible. Exothermic reaction involves release in the energy which makes the reaction products stable.
-Among the options given, all are metals. So the best reagent is checked by the value of ionization energy and the valency also. We need to liberate 1 molecule of halogen and for that, metal with valency 2 should be preferred.
-Zn is the only metal here with valency 2. Also it has very less value of ionization energy and so it results in the exothermic process. This is mandatory as the formation of double bonds is an endothermic process and the overall reaction has to be exothermic.
Therefore the correct option is A. Zn
Reaction for dehalogenation can be shown as

Note: Dehalogenation is different from dehydrohalogenation. In the former, the halogen escapes as a gas and in the form of its molecule with the formula ${{X}_{2}}$ where X represents the halogens. In the latter, HX gas is liberated after the completion of the reaction where X is the single atom of halogen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




