
The reaction of formic acid with concentrated sulphuric acid gives:
A) \[C{O_2}\]
B) \[CO\]
C) Oxalic acid
D) Acetic acid
Answer
584.4k+ views
Hint: We know that concentrated sulphuric acid is a dehydrating agent, and removes water molecules from any substance.
The formula of formic acid is \[HCOOH\] , which is the first member and basic acid in the carboxylic acid group.
The final result will be dehydrated formic acid, which is gas and it is toxic gas. Sulphuric acid will get hydrated, and it does not undergo any change, it remains unaffected and also acts as catalyst.
Complete answer:
Generally, we know that acid and base react to give salt and water, but reaction of 2 acids is not seen. But this reaction can happen if the acid is a strong oxidising agent as is sulphuric acid.
When formic acid, \[HCOOH\] is reacting with concentrated Sulphuric acid then the formic acid will be dehydrated and sulphuric acid will be hydrated.
The reaction can be written as follows:
$HCOOH + conc.{H_2}S{O_4} \to CO + {H_2}S{O_4}.{H_2}O$
Here, sulphuric acid will not react but act as catalyst and just help in dehydrating formic acid, to form Carbon monoxide and hydrated sulphuric acid. The gas evolved, carbon monoxide is
Formic acid is also called methanoic acid. The structure of Formic acid is shown below:
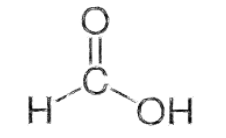
Thus, the correct answer is (B) \[CO\].
Additional information: Formic Acid is the simplest compound in the group of carboxylic acid. The word formic means ant, as this acid is present in ant sting. The IUPAC or chemical name of Formic acid is methanoic acid.
Carbon Monoxide is toxic gas and inhalation of this gas can lead to even death of a person.
Incomplete combustion of hydrocarbons and fuel also release carbon monoxide gas. Even incomplete combustion of fuel in vehicles also releases this toxic gas.
Note: Concentrated sulphuric acid is highly corrosive. It has to be handled carefully. Concentrated sulphuric acid has a highly dehydrating property and also it acts as oxidising agent. But in the above reaction it acts as a dehydrating agent, by dehydrating formic acid to form Carbon monoxide.
The formula of formic acid is \[HCOOH\] , which is the first member and basic acid in the carboxylic acid group.
The final result will be dehydrated formic acid, which is gas and it is toxic gas. Sulphuric acid will get hydrated, and it does not undergo any change, it remains unaffected and also acts as catalyst.
Complete answer:
Generally, we know that acid and base react to give salt and water, but reaction of 2 acids is not seen. But this reaction can happen if the acid is a strong oxidising agent as is sulphuric acid.
When formic acid, \[HCOOH\] is reacting with concentrated Sulphuric acid then the formic acid will be dehydrated and sulphuric acid will be hydrated.
The reaction can be written as follows:
$HCOOH + conc.{H_2}S{O_4} \to CO + {H_2}S{O_4}.{H_2}O$
Here, sulphuric acid will not react but act as catalyst and just help in dehydrating formic acid, to form Carbon monoxide and hydrated sulphuric acid. The gas evolved, carbon monoxide is
Formic acid is also called methanoic acid. The structure of Formic acid is shown below:

Thus, the correct answer is (B) \[CO\].
Additional information: Formic Acid is the simplest compound in the group of carboxylic acid. The word formic means ant, as this acid is present in ant sting. The IUPAC or chemical name of Formic acid is methanoic acid.
Carbon Monoxide is toxic gas and inhalation of this gas can lead to even death of a person.
Incomplete combustion of hydrocarbons and fuel also release carbon monoxide gas. Even incomplete combustion of fuel in vehicles also releases this toxic gas.
Note: Concentrated sulphuric acid is highly corrosive. It has to be handled carefully. Concentrated sulphuric acid has a highly dehydrating property and also it acts as oxidising agent. But in the above reaction it acts as a dehydrating agent, by dehydrating formic acid to form Carbon monoxide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




