
The reaction of ethanol with conc. ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$gives:
A. ethane
B. ethene
C. ethyne
D. ethanoic acid
Answer
592.8k+ views
Hint: The reaction between ethanol and conc. ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ is a dehydration reaction. Dehydration means removal of water molecules to form alkenes. As concentrated ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ is a dehydrating agent. Ethanol has two carbon atoms and an alcohol group and rest vacancies are filled by hydrogen atoms.
Complete answer:
Let us form the product of this reaction using the mechanism:
Step (1)- Attack of ${{\text{H}}^{+}}$ ion on the lone pair of oxygen atom, as the ${{\text{H}}^{+}}$ ion is electron deficient and lone pairs are electron rich. The ${{\text{H}}^{+}}$ ion is formed from dissociation of sulphuric acid into its respective ions $\left( \text{2}{{\text{H}}^{+}}+\text{SO}_{4}^{2-} \right)$. This attack on lone pairs leads to formation of protonated alcohol.
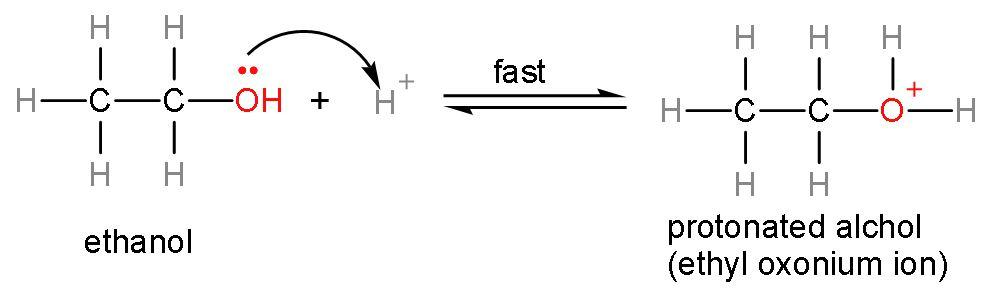
Step (2)- Removal of water occurs from the protonated alcohol to form carbocation. This is a slow step or rate-determining step. The rate of any reaction is determined by the slowest step.
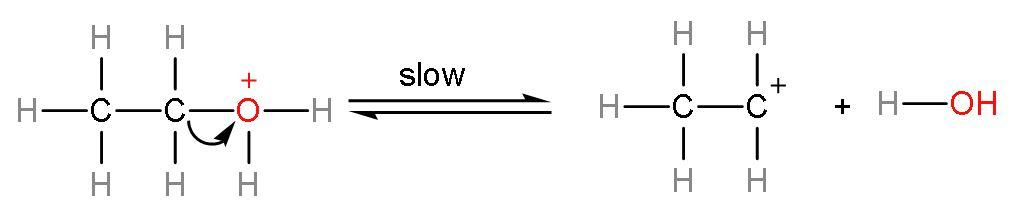
Step (3)- Formation of alkene or ethene takes place by the elimination of ${{\text{H}}^{+}}$ion from the carbocation. The ${{\text{H}}^{+}}$ion is a catalyst of this reaction as it remains unutilized.
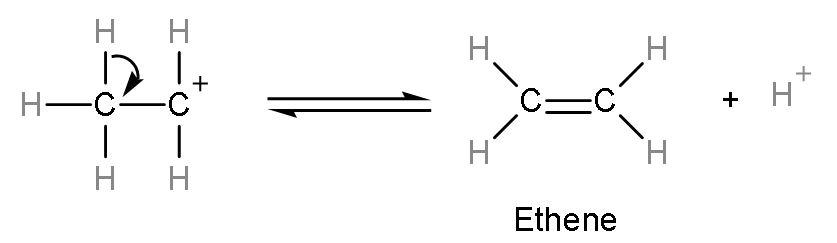
The correct answer to this question is ethene is formed as a product on reaction between ethanol and conc. ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ So, the correct answer is “Option B”.
Additional Information:
Secondary and tertiary alcohols dehydrate under mild conditions only because after protonation, the carbocation formed is highly stable due to hyperconjugation. Thus, the relative ease of dehydration of alcohols is $\text{Tertiary}>\text{Secondary}>\text{Primary}$.
Note: Alcohols reacts with conc.${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ not only to form alkenes but at different temperatures, the reactions are different thus, the products formed are different. Like,
(1) At 383 K, ethanol reacts with sulphuric acid to form ethyl hydrogen sulphate and water.
${{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}+\text{conc}\text{.}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\xrightarrow{383\text{K}}{{\text{C}}_{2}}{{\text{H}}_{5}}\text{HS}{{\text{O}}_{4}}+{{\text{H}}_{2}}\text{O}$
(2) At 413 K, ethanol reacts with sulphuric acid to form diethyl ether. As, the two molecules of ethanol combine to remove water.
${{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}+\text{conc}\text{.}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\xrightarrow{413\text{K}}{{\text{C}}_{2}}{{\text{H}}_{5}}{\mathrm O}{{\text{C}}_{2}}{{\text{H}}_{5}}+{{\eta }_{2}}{\mathrm O}$
(3) At 443 K, ethanol reacts with sulphuric acid to form ethene. Ethanol gets protonated and water gets removed.
${{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}+\text{conc}\text{.}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\xrightarrow{443\text{K}}{{\text{C}}_{2}}{{\text{H}}_{4}}+{{\eta }_{2}}{\mathrm O}$
So, the product formed depends on the reagents and as well as on the temperatures.
Complete answer:
Let us form the product of this reaction using the mechanism:
Step (1)- Attack of ${{\text{H}}^{+}}$ ion on the lone pair of oxygen atom, as the ${{\text{H}}^{+}}$ ion is electron deficient and lone pairs are electron rich. The ${{\text{H}}^{+}}$ ion is formed from dissociation of sulphuric acid into its respective ions $\left( \text{2}{{\text{H}}^{+}}+\text{SO}_{4}^{2-} \right)$. This attack on lone pairs leads to formation of protonated alcohol.

Step (2)- Removal of water occurs from the protonated alcohol to form carbocation. This is a slow step or rate-determining step. The rate of any reaction is determined by the slowest step.

Step (3)- Formation of alkene or ethene takes place by the elimination of ${{\text{H}}^{+}}$ion from the carbocation. The ${{\text{H}}^{+}}$ion is a catalyst of this reaction as it remains unutilized.

The correct answer to this question is ethene is formed as a product on reaction between ethanol and conc. ${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ So, the correct answer is “Option B”.
Additional Information:
Secondary and tertiary alcohols dehydrate under mild conditions only because after protonation, the carbocation formed is highly stable due to hyperconjugation. Thus, the relative ease of dehydration of alcohols is $\text{Tertiary}>\text{Secondary}>\text{Primary}$.
Note: Alcohols reacts with conc.${{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ not only to form alkenes but at different temperatures, the reactions are different thus, the products formed are different. Like,
(1) At 383 K, ethanol reacts with sulphuric acid to form ethyl hydrogen sulphate and water.
${{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}+\text{conc}\text{.}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\xrightarrow{383\text{K}}{{\text{C}}_{2}}{{\text{H}}_{5}}\text{HS}{{\text{O}}_{4}}+{{\text{H}}_{2}}\text{O}$
(2) At 413 K, ethanol reacts with sulphuric acid to form diethyl ether. As, the two molecules of ethanol combine to remove water.
${{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}+\text{conc}\text{.}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\xrightarrow{413\text{K}}{{\text{C}}_{2}}{{\text{H}}_{5}}{\mathrm O}{{\text{C}}_{2}}{{\text{H}}_{5}}+{{\eta }_{2}}{\mathrm O}$
(3) At 443 K, ethanol reacts with sulphuric acid to form ethene. Ethanol gets protonated and water gets removed.
${{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}+\text{conc}\text{.}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\xrightarrow{443\text{K}}{{\text{C}}_{2}}{{\text{H}}_{4}}+{{\eta }_{2}}{\mathrm O}$
So, the product formed depends on the reagents and as well as on the temperatures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




