
The reaction of cyclooctyne with $HgS{O_4}$ in the presence of aqueous ${H_2}S{O_4}$gives,
A.
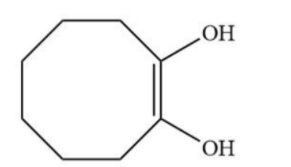
B.
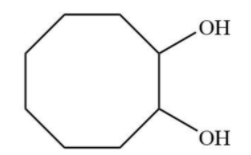
C.
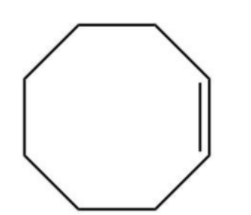
D.
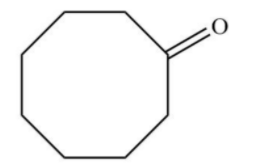




Answer
582.6k+ views
Hint: -The reaction of alkyne and \[HgS{O_4}\] in the presence of aqueous \[{H_2}S{O_4}\] gives carbonyl compounds ,it may be aldehyde or may be ketone .This reaction is also known as Kucherov reaction.
Now. Let’s discuss the general Kucherov reaction which is shown below.
Complete step by step solution
Due to the presence of two pi-bonds, alkynes have a very low tendency for electrophilic addition than alkenes. But with the help of various promoter catalysts such as $HgS{O_4}$ and $CdS{O_4}$ electrophilic addition can take place.
Let us see the mechanism for the general reaction of alkyne with $HgS{O_4}$ in the presence of aqueous \[{H_2}S{O_4}\] ;
In the first step, protonation of alkyne will take place by the $HgS{O_4}$ and nucleophile ${H_2}0$ which will react with the pi-bond of the alkyne. After the attack both removal of hydrogen ions will take place and there will be removal of $HgS{O_4}$ using acidic work up. Then enol will be formed. Then these enols get tautomerized to ketone which is the desired product.
Keto-enol tautomerism is a very famous and they interconverted using acid or base. Normally keto form compounds are more stable than the enol form compound. But there are few examples where keto form is also very stable.
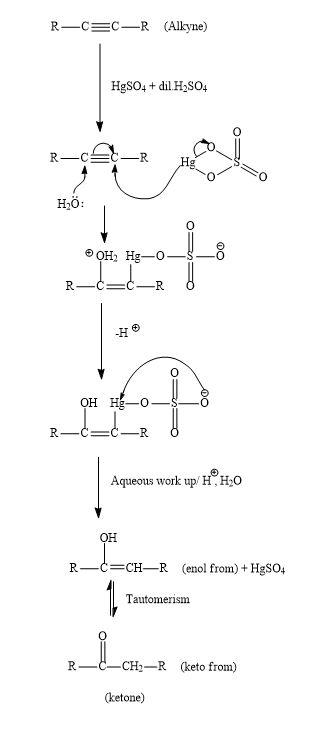
Similarly, when cyclooctyne reacts then there will be a formation of enol which gets ketomerises to keto form as keto form is more stable.
The reaction that takes place is;
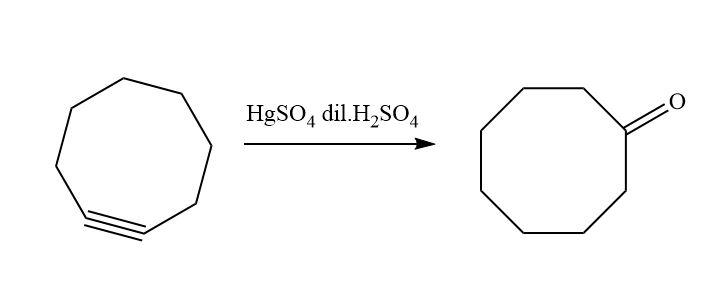
Hence, option D is correct.
Note:
With the same mechanism, when alkenes are treated with the same reagents then there will be the formation of the corresponding alcohol.
Now. Let’s discuss the general Kucherov reaction which is shown below.
Complete step by step solution
Due to the presence of two pi-bonds, alkynes have a very low tendency for electrophilic addition than alkenes. But with the help of various promoter catalysts such as $HgS{O_4}$ and $CdS{O_4}$ electrophilic addition can take place.
Let us see the mechanism for the general reaction of alkyne with $HgS{O_4}$ in the presence of aqueous \[{H_2}S{O_4}\] ;
In the first step, protonation of alkyne will take place by the $HgS{O_4}$ and nucleophile ${H_2}0$ which will react with the pi-bond of the alkyne. After the attack both removal of hydrogen ions will take place and there will be removal of $HgS{O_4}$ using acidic work up. Then enol will be formed. Then these enols get tautomerized to ketone which is the desired product.
Keto-enol tautomerism is a very famous and they interconverted using acid or base. Normally keto form compounds are more stable than the enol form compound. But there are few examples where keto form is also very stable.

Similarly, when cyclooctyne reacts then there will be a formation of enol which gets ketomerises to keto form as keto form is more stable.
The reaction that takes place is;

Hence, option D is correct.
Note:
With the same mechanism, when alkenes are treated with the same reagents then there will be the formation of the corresponding alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




