
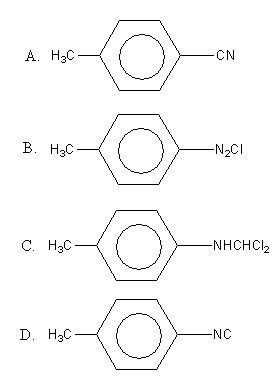
The reaction of chloroform with alcoholic KOH with p-toluidine form

Answer
560.7k+ views
Hint: The reagent chloroform with alcoholic KOH is used to convert the amine functional group into cyanide. The reaction is known as the Carbylamine reaction. In this reaction, isocyanide forms so, to determine the answer we should know the structure of isocyanide.
Complete step-by-step answer:
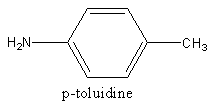
The structure of p-toluidine is as follows:
The alcoholic potassium hydroxide abstract the hydrogen from chloroform and form dichlorocarbene. The nitrogen of p-toluidine has lone pairs so it attacks on the dichlorocarbene and gets attached. Then one proton forms amine group transfer to the dichlorocarbene group. Then hydrogen from amine and chlorine from the dichlorocarbene group removes forming a C-N double bond. Again the base abstract the second proton from amine and a triple bond forms between amine’s nitrogen and carbene’s carbon by the removal of chlorine.
The reaction is shown as follows:
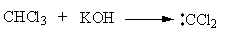
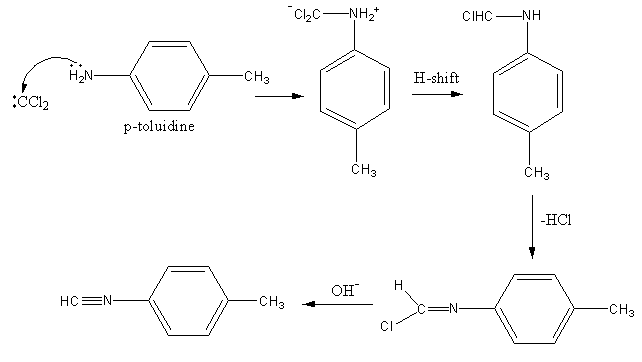
So, the reaction of chloroform with alcoholic KOH with p-toluidine form para-isocyano methyl benzene.
Therefore, option (D), is the correct answer.
Note: The reagent chloroform with base such as KOH or NaOH is used for the conversion of the amine functional group into isocyanides. The conversion of the amine functional group into the isocyanides functional group by the reaction of a primary amine with chloroform and base is known as the Carbylamine reaction. Only primary amine gives the Carbylamine reaction so this reaction is used to differentiate amines. So, the Carbylamine reaction is known as the Carbylamine test. The reaction is used to differentiate the primary amine from secondary and tertiary amines.
Complete step-by-step answer:
The structure of p-toluidine is as follows:

The alcoholic potassium hydroxide abstract the hydrogen from chloroform and form dichlorocarbene. The nitrogen of p-toluidine has lone pairs so it attacks on the dichlorocarbene and gets attached. Then one proton forms amine group transfer to the dichlorocarbene group. Then hydrogen from amine and chlorine from the dichlorocarbene group removes forming a C-N double bond. Again the base abstract the second proton from amine and a triple bond forms between amine’s nitrogen and carbene’s carbon by the removal of chlorine.
The reaction is shown as follows:
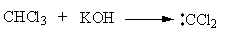

So, the reaction of chloroform with alcoholic KOH with p-toluidine form para-isocyano methyl benzene.
Therefore, option (D), is the correct answer.
Note: The reagent chloroform with base such as KOH or NaOH is used for the conversion of the amine functional group into isocyanides. The conversion of the amine functional group into the isocyanides functional group by the reaction of a primary amine with chloroform and base is known as the Carbylamine reaction. Only primary amine gives the Carbylamine reaction so this reaction is used to differentiate amines. So, the Carbylamine reaction is known as the Carbylamine test. The reaction is used to differentiate the primary amine from secondary and tertiary amines.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




