
The reaction of 1 mole each of p-hydroxyacetophenone and methyl magnesium iodide will give:
Answer
562.8k+ views
Hint: Active hydrogen produces alkane on reaction with methyl magnesium iodide as it is a type of Grignard Reagent. The Grignard Reagents are formed by the reaction of magnesium metal with alkyl halides.
Complete Step by step Solution:
Methyl magnesium iodide is $C{{H}_{3}}MgI$
p- Hydroxyacetophenone is
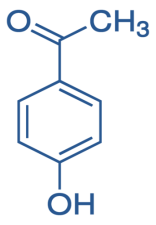
So, reaction of p-hydroxyacetophenone and Methyl magnesium iodide gives-
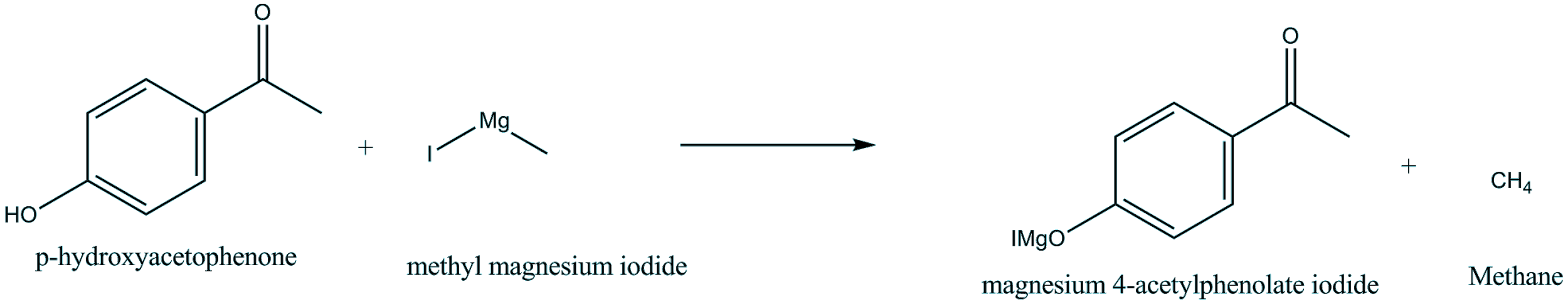
The active hydrogen is present in p-hydroxyacetophenone which produces alkane ($C{{H}_{4}}$ ) on reaction with Grignard reagent ($C{{H}_{3}}MgI$). So, the H atom of hydroxyl group in p-hydroxyacetophenone is replaced by Mg-I. The methyl of $C{{H}_{3}}MgI$ and active H of another compound combines to form methane ($C{{H}_{4}}$).
Additional Information: Grignard Reagent: It is a chemical compound with the generic formula R$-$Mg$-$X, where X is a halogen and R is an organic group, namely an alkyl or aryl. The two typical examples of Grignard Reagents are methyl magnesium chloride and phenyl magnesium bromide. These are the popular reagents in organic synthesis for creating new carbon-carbon bonds.
Pure Grignard reagents are extremely reactive solids. Grignard reagent is a key step in the industrial production of Tamoxifen which is currently used for the treatment of estrogen receptor positive breast cancer in women.
Para position: p-hydroxyl in the above given compound means that hydroxyl group i.e. OH group is present at the Para position. Para position is the one in which there are two functional groups tied to a ring of benzene in the position 1 and 4 of the ring.
There is one more position Ortho, in which the substituents occupy positions next to each other, which may be numbered 1 and 2. Meta position contains substituents at position 1 and3. Ortho and Para are more stable as compared to Meta position.
Note: The knowledge of Para, Ortho and Meta position should be known to one. The Grignard reaction is an organometallic chemical reaction in which alkyl, ally, vinyl, or aryl-magnesium halides (Grignard reagent) add to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds
Complete Step by step Solution:
Methyl magnesium iodide is $C{{H}_{3}}MgI$
p- Hydroxyacetophenone is

So, reaction of p-hydroxyacetophenone and Methyl magnesium iodide gives-

The active hydrogen is present in p-hydroxyacetophenone which produces alkane ($C{{H}_{4}}$ ) on reaction with Grignard reagent ($C{{H}_{3}}MgI$). So, the H atom of hydroxyl group in p-hydroxyacetophenone is replaced by Mg-I. The methyl of $C{{H}_{3}}MgI$ and active H of another compound combines to form methane ($C{{H}_{4}}$).
Additional Information: Grignard Reagent: It is a chemical compound with the generic formula R$-$Mg$-$X, where X is a halogen and R is an organic group, namely an alkyl or aryl. The two typical examples of Grignard Reagents are methyl magnesium chloride and phenyl magnesium bromide. These are the popular reagents in organic synthesis for creating new carbon-carbon bonds.
Pure Grignard reagents are extremely reactive solids. Grignard reagent is a key step in the industrial production of Tamoxifen which is currently used for the treatment of estrogen receptor positive breast cancer in women.
Para position: p-hydroxyl in the above given compound means that hydroxyl group i.e. OH group is present at the Para position. Para position is the one in which there are two functional groups tied to a ring of benzene in the position 1 and 4 of the ring.
There is one more position Ortho, in which the substituents occupy positions next to each other, which may be numbered 1 and 2. Meta position contains substituents at position 1 and3. Ortho and Para are more stable as compared to Meta position.
Note: The knowledge of Para, Ortho and Meta position should be known to one. The Grignard reaction is an organometallic chemical reaction in which alkyl, ally, vinyl, or aryl-magnesium halides (Grignard reagent) add to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




