
The reaction
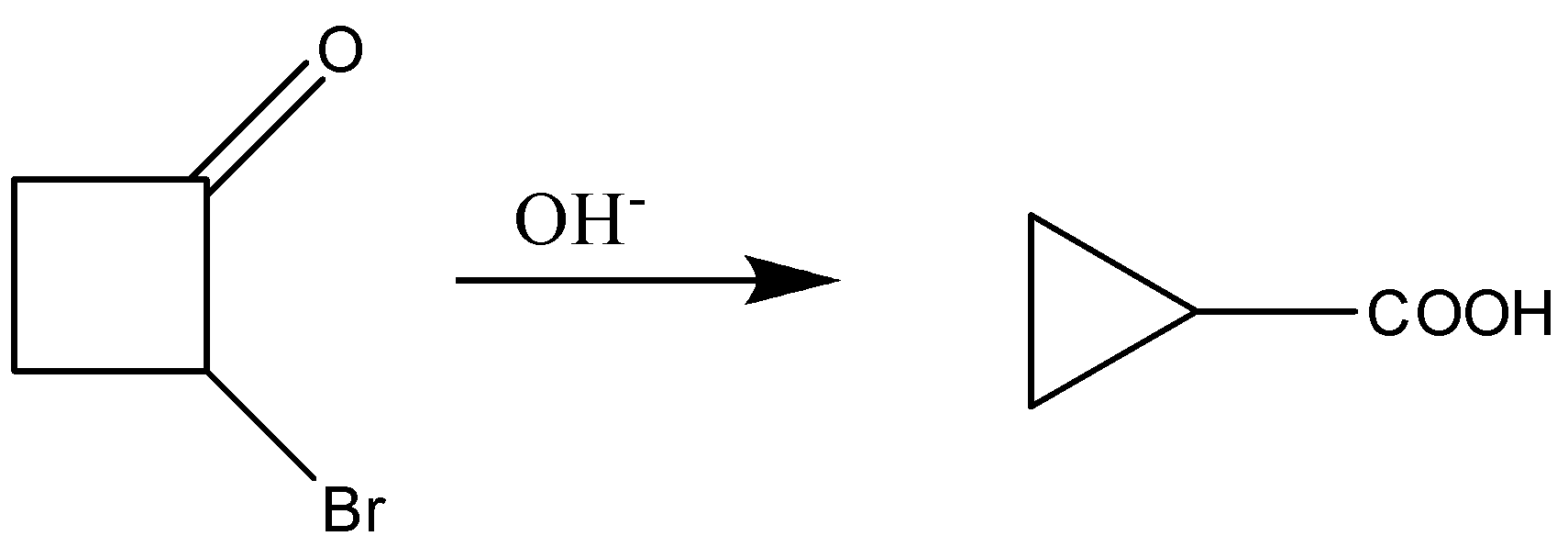
Is an example of:
A) Wolff rearrangement
B) Favorskii rearrangement
C) Stevens rearrangement
D) Wagner-Meerwein

Answer
558.9k+ views
Hint: In the diagram given above we can see that there is conversion of $\alpha$-halo ketones into the acid which is carboxylic acid through the arrangement of the atoms of the compounds and there is contraction of the ring.
Complete step by step answer:
Now we will study about each rearrangement process given and will select the correct option:
The Favorskii rearrangement is most principally a rearrangement reaction which forms the carboxylic acid derivatives from cyclopropanes and $\alpha$-halo ketones which leads. Favorskii rearrangement constitutes a ring contraction in case of cyclic $\alpha$-halo ketones.
In chemistry; The Wolff rearrangement is the rearrangement reaction in which there is a conversion reaction of $\alpha$-diazocarbonyl compounds forming into a ketene which occur by loss of dinitrogen followed by 1,2-rearrangement. Ketene formed in wolf's rearrangement is an intermediate product, which on addition with weakly acidic nucleophiles such as water, alcohols, and amines can undergo nucleophilic attack, undergo [2+2] cycloaddition reactions forming four-membered rings or to generate carboxylic acid derivatives.
In organic chemistry, The Stevens rearrangement is the rearrangement reaction which converts quaternary ammonium and sulfonium salts to their corresponding sulfides or amines in a 1,2-rearrangement taking place in the presence of a strong base in. These reactants can be obtained by alternative method alkylation of the amines & sulfides. An electron-withdrawing group R is present next to Amine Methylene Bridge.
In chemistry Wagner–Meerwein rearrangement is the rearrangement reaction where carbocation 1,2-rearrangement reactions take place in which there is migration of hydrogen, aryl or alkyl from one carbon to their neighbouring carbon.
Thus, option B is the correct answer.
Note: This rearrangement takes place within the presence of a base, sometimes hydroxide, to yield an acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. $\alpha$,$\alpha$’-Dihaloketones eliminate HX under the certain reaction conditions to offer $\alpha$,β-unsaturated carbonyl compounds.
Complete step by step answer:
Now we will study about each rearrangement process given and will select the correct option:
The Favorskii rearrangement is most principally a rearrangement reaction which forms the carboxylic acid derivatives from cyclopropanes and $\alpha$-halo ketones which leads. Favorskii rearrangement constitutes a ring contraction in case of cyclic $\alpha$-halo ketones.
In chemistry; The Wolff rearrangement is the rearrangement reaction in which there is a conversion reaction of $\alpha$-diazocarbonyl compounds forming into a ketene which occur by loss of dinitrogen followed by 1,2-rearrangement. Ketene formed in wolf's rearrangement is an intermediate product, which on addition with weakly acidic nucleophiles such as water, alcohols, and amines can undergo nucleophilic attack, undergo [2+2] cycloaddition reactions forming four-membered rings or to generate carboxylic acid derivatives.
In organic chemistry, The Stevens rearrangement is the rearrangement reaction which converts quaternary ammonium and sulfonium salts to their corresponding sulfides or amines in a 1,2-rearrangement taking place in the presence of a strong base in. These reactants can be obtained by alternative method alkylation of the amines & sulfides. An electron-withdrawing group R is present next to Amine Methylene Bridge.
In chemistry Wagner–Meerwein rearrangement is the rearrangement reaction where carbocation 1,2-rearrangement reactions take place in which there is migration of hydrogen, aryl or alkyl from one carbon to their neighbouring carbon.
Thus, option B is the correct answer.
Note: This rearrangement takes place within the presence of a base, sometimes hydroxide, to yield an acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. $\alpha$,$\alpha$’-Dihaloketones eliminate HX under the certain reaction conditions to offer $\alpha$,β-unsaturated carbonyl compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




