
The reaction intermediate produced by hemolytic cleavage of a bond is called:
A.Carbene
B.Carbocation
C.Carbanion
D.Free radical
Answer
591.3k+ views
Hint: Homolytic cleavage is the breaking of a covalent bond in such a way that each fragment gets one of the shared electrons and each atom shares electrons with itself.
Complete step by step answer:
The reaction intermediate produced by homolytic cleavage of bonds is called free radical.
The covalent bond is cleaved in such a manner that each atom shares electrons with itself and free radical is formed.
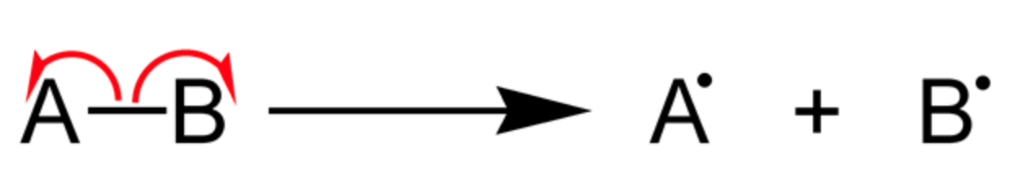
Free radicals are the reaction intermediates formed containing carbon such that carbon gets an unpaired electron. In this process each atom takes away one of the two electrons forming a single covalent bond. It will further produce two new species.
This process is also known as radical fission and the energy required for homolytic cleavage is bond-dissociation energy.
Hence, option D is correct.
Note:
Free radicals, molecules (or atoms) having one or more unpaired electrons are recognized as important short-lived, highly reactive intermediates in a variety of organic, and some inorganic reactions. These are derived either from normal metabolic processes in the human body or from external sources such as exposure to X-rays, ozone, industrial chemicals.
Complete step by step answer:
The reaction intermediate produced by homolytic cleavage of bonds is called free radical.
The covalent bond is cleaved in such a manner that each atom shares electrons with itself and free radical is formed.

Free radicals are the reaction intermediates formed containing carbon such that carbon gets an unpaired electron. In this process each atom takes away one of the two electrons forming a single covalent bond. It will further produce two new species.
This process is also known as radical fission and the energy required for homolytic cleavage is bond-dissociation energy.
Hence, option D is correct.
Note:
Free radicals, molecules (or atoms) having one or more unpaired electrons are recognized as important short-lived, highly reactive intermediates in a variety of organic, and some inorganic reactions. These are derived either from normal metabolic processes in the human body or from external sources such as exposure to X-rays, ozone, industrial chemicals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




