
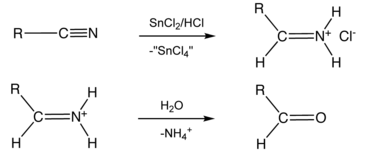
The reaction \[{\text{RCN}}\xrightarrow{{{\text{SnC}}{{\text{l}}_{\text{2}}}{\text{/HCl}}}}{\text{(A)}}\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{RCHO + N}}{{\text{H}}_{\text{4}}}{\text{Cl}}\] is known as:
A Reformatsky reaction
B Stephen's reaction
C Tishchenko reaction
D Cannizzaro reaction
Answer
589.5k+ views
Hint: Reagent can be a substance or mixture of compounds which are basically used in chemical analysis or reactions. When a reagent is added to a system it causes chemical reactions. For a particular reaction, a particular reagent gets consumed in the process of the chemical reaction.
Complete step by step answer:
There are so many different kinds of reaction sequence pathways to do this conversion. The uses of every reagents which are used in conversions should be known. Always find out the change of groups and make a suitable reaction sequence for conversions.Name reactions are the chemical reactions which are named by the developers of that particular reaction. For example, Reformatsky reaction, Cannizzaro reaction, Tishchenko reaction etc.
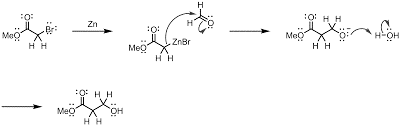
Now, the Reformatsky reaction is shown below. in this reaction from the α-halo ester in the presence of zinc to give a β-hydroxy ester is formed.
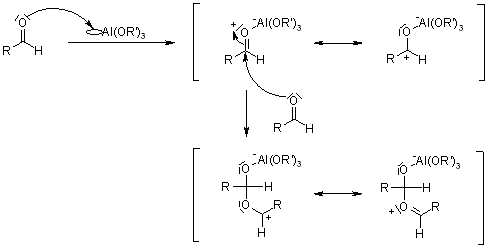
The Tishchenko reaction is shown below, in this reaction from aldehyde or ketone ester is formed.
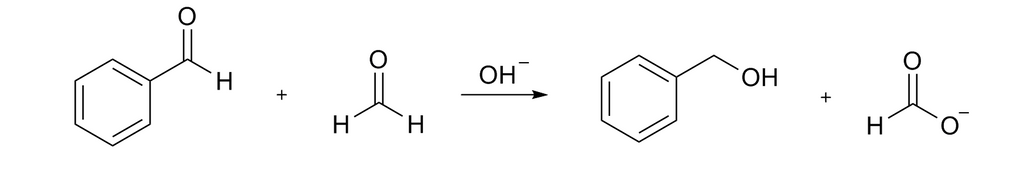
The Cannizzaro reaction is shown below, in this reaction oxidation of one carbonyl group and reduction of another carbonyl group happens to form acid and alcohol respectively.
Stephen's reaction is shown below, in this reaction from alkyl cyanide aldehyde is formed.
Therefore the correct answer is B.
Note:
Another name reaction is the Friedel-Craft reaction. This reaction is basically a coupling reaction between aromatic compound (benzene) and alkyl halide or acyl halide in presence of anhydrous Lewis acid \[{\text{AlC}}{{\text{l}}_{\text{3}}}\]. In presence of aq. \[{\text{AlC}}{{\text{l}}_{\text{3}}}\] it reduces the nucleophilicity of the benzene ring by formation of cation of benzene ring.
Complete step by step answer:
There are so many different kinds of reaction sequence pathways to do this conversion. The uses of every reagents which are used in conversions should be known. Always find out the change of groups and make a suitable reaction sequence for conversions.Name reactions are the chemical reactions which are named by the developers of that particular reaction. For example, Reformatsky reaction, Cannizzaro reaction, Tishchenko reaction etc.
Now, the Reformatsky reaction is shown below. in this reaction from the α-halo ester in the presence of zinc to give a β-hydroxy ester is formed.

The Tishchenko reaction is shown below, in this reaction from aldehyde or ketone ester is formed.

The Cannizzaro reaction is shown below, in this reaction oxidation of one carbonyl group and reduction of another carbonyl group happens to form acid and alcohol respectively.

Stephen's reaction is shown below, in this reaction from alkyl cyanide aldehyde is formed.

Therefore the correct answer is B.
Note:
Another name reaction is the Friedel-Craft reaction. This reaction is basically a coupling reaction between aromatic compound (benzene) and alkyl halide or acyl halide in presence of anhydrous Lewis acid \[{\text{AlC}}{{\text{l}}_{\text{3}}}\]. In presence of aq. \[{\text{AlC}}{{\text{l}}_{\text{3}}}\] it reduces the nucleophilicity of the benzene ring by formation of cation of benzene ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




