
The ratio of σ and π bonds in benzene is :
a.) 4 : 1
b.) 6 : 1
c.) 4 : 3
d.) 8 : 1
Answer
576k+ views
Hint: The sigma bond is the single bond made between two atoms by equal sharing of two electrons. The pi bond is the second bond that is made between two atoms.
The first bond made is always the sigma while the second and then third both are counted as pi bonds. From following structure, we can count the number of bonds and find their ratio as-
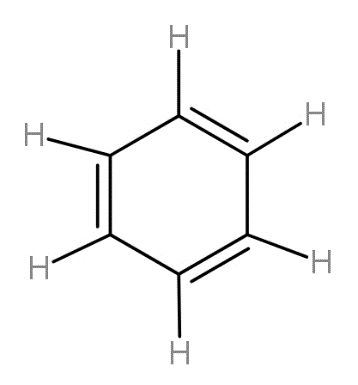
Complete step by step answer:
We know that benzene is an aromatic molecule. It has a molecular formula ${C_6}{H_6}$.
The sigma bond is the single bond made between two atoms by equal sharing of two electrons. The pi bond is the second bond that is made between two atoms.
The first bond made is always the sigma while the second and then third both are counted as pi bonds.
For counting the number of sigma and pi bonds, we should know the structure of the benzene ring. This six carbon ring has structure as -

The carbon-hydrogen bonds are all sigma bonds and the first carbon-carbon bond is sigma bond while the second carbon-carbon bond is pi bond.
So, if we count the number of bonds of each type, we have
Number of sigma bonds = 12
Number of pi bonds = 3
Thus, the ratio is as -
$\dfrac{{Number{\text{ of sigma bonds}}}}{{Number{\text{ of pi bonds}}}}$ = $\dfrac{{12}}{3}$
$\dfrac{{Number{\text{ of sigma bonds}}}}{{Number{\text{ of pi bonds}}}}$ = 4 : 1
So, the correct answer is “Option A”.
Note: It must be noted that the both sigma and pi bonds are covalent in nature. These both are formed by equal sharing of electrons. However, it is possible that if one of the atoms is more electronegative, then it will attract the shared pair more towards itself.
The first bond made is always the sigma while the second and then third both are counted as pi bonds. From following structure, we can count the number of bonds and find their ratio as-

Complete step by step answer:
We know that benzene is an aromatic molecule. It has a molecular formula ${C_6}{H_6}$.
The sigma bond is the single bond made between two atoms by equal sharing of two electrons. The pi bond is the second bond that is made between two atoms.
The first bond made is always the sigma while the second and then third both are counted as pi bonds.
For counting the number of sigma and pi bonds, we should know the structure of the benzene ring. This six carbon ring has structure as -

The carbon-hydrogen bonds are all sigma bonds and the first carbon-carbon bond is sigma bond while the second carbon-carbon bond is pi bond.
So, if we count the number of bonds of each type, we have
Number of sigma bonds = 12
Number of pi bonds = 3
Thus, the ratio is as -
$\dfrac{{Number{\text{ of sigma bonds}}}}{{Number{\text{ of pi bonds}}}}$ = $\dfrac{{12}}{3}$
$\dfrac{{Number{\text{ of sigma bonds}}}}{{Number{\text{ of pi bonds}}}}$ = 4 : 1
So, the correct answer is “Option A”.
Note: It must be noted that the both sigma and pi bonds are covalent in nature. These both are formed by equal sharing of electrons. However, it is possible that if one of the atoms is more electronegative, then it will attract the shared pair more towards itself.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




