
The radical given below is aromatic, because it has
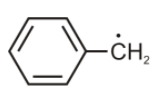
A. 7 p-orbitals and 6 unpaired electrons
B. 7 p- orbitals and 7 unpaired electrons
C. 6 p-orbitals and 7 unpaired electrons
D. 6 p-orbitals and 1 unpaired electron

Answer
566.4k+ views
Hint: Aromaticity is a chemical property of organic compounds, aromatic compounds follow Huckel’s rule. They have a high degree of stability. They show electrophilic substitution reactions. There is a diamagnetic current in these compounds.
Complete Step by step answer: aromatic compounds have high degree of stability due to filled bonding molecular orbital.
They follow huckel’s rule of aromaticity-
Huckel’s rule states that, for a compound to be aromatic, the compound should be planar, cyclic and should have (4n+2) pi electrons that should be in continuous delocalization or an uninterrupted cyclic pi electron cloud should be there. Here n= 0,1,2……
So if we put n=0 ,we get 2 pi electrons
If we put n=1, we get 6 pi electrons
If we put n=2, we get 10 pi electrons and so on
For example benzene, we all know are aware of the structure of benzene. It is planar, it is cyclic and it has 6 Pi electrons
In the above case, it is aromatic, because it is planar, cyclic and has 6 pi electrons which are in continuous delocalization. And hence satisfying the condition of Huckle’s rule of aromaticity.
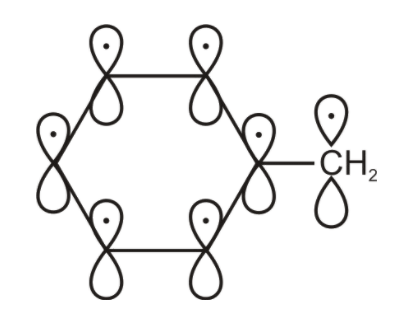
But the free radical also has 1 unpaired electron and 1 p orbital. Here the free radical unpaired electron is also involved in the delocalization. So total we have 7 unpaired electrons 7 p- orbitals.
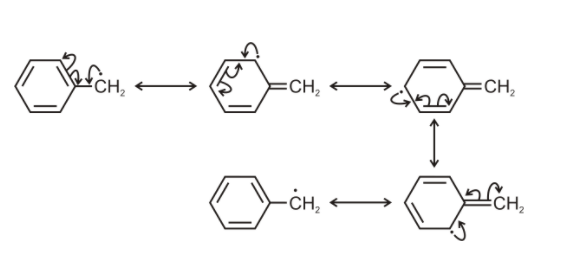
Hence the correct option will be B. total 7 unpaired electrons and 7 p- orbitals.
Note: Molecules which are cyclic, planar, and have 4n pi electrons which are in conjugation are called as anti-aromatic. For example: cyclobuta-1,3-diene. The compounds which do not follow huckel’s rule for aromaticity and anti-aromaticity are non-aromatic. For example: cyclooctatetraene.
Complete Step by step answer: aromatic compounds have high degree of stability due to filled bonding molecular orbital.
They follow huckel’s rule of aromaticity-
Huckel’s rule states that, for a compound to be aromatic, the compound should be planar, cyclic and should have (4n+2) pi electrons that should be in continuous delocalization or an uninterrupted cyclic pi electron cloud should be there. Here n= 0,1,2……
So if we put n=0 ,we get 2 pi electrons
If we put n=1, we get 6 pi electrons
If we put n=2, we get 10 pi electrons and so on
For example benzene, we all know are aware of the structure of benzene. It is planar, it is cyclic and it has 6 Pi electrons
In the above case, it is aromatic, because it is planar, cyclic and has 6 pi electrons which are in continuous delocalization. And hence satisfying the condition of Huckle’s rule of aromaticity.

But the free radical also has 1 unpaired electron and 1 p orbital. Here the free radical unpaired electron is also involved in the delocalization. So total we have 7 unpaired electrons 7 p- orbitals.

Hence the correct option will be B. total 7 unpaired electrons and 7 p- orbitals.
Note: Molecules which are cyclic, planar, and have 4n pi electrons which are in conjugation are called as anti-aromatic. For example: cyclobuta-1,3-diene. The compounds which do not follow huckel’s rule for aromaticity and anti-aromaticity are non-aromatic. For example: cyclooctatetraene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




