
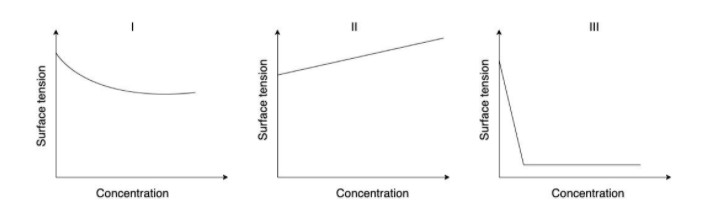
The qualitative sketches I, II and III given below show the variation of surface tension with molar concentration of three different aqueous solutions of $KCl,\,C{H_3}OH$ and $C{H_3}{(C{H_2})_{11}}OSO_3^ - N{a^ + }$ at room temperature.
The correct assignment of the sketches is:
A. $I:KCl;\,II:\,C{H_3}OH;\,\,III:C{H_3}{(C{H_2})_{11}}OSO_3^ - N{a^ + }$
B. $I:C{H_3}{(C{H_2})_{11}}OSO_3^ - N{a^ + };\,II:\,C{H_3}OH;\,\,III:\,KCl$
C. $I:KCl;\,II:\,C{H_3}{(C{H_2})_{11}}OSO_3^ - N{a^ + };\,\,III:C{H_3}OH$
D. $I:C{H_3}OH;\,II:\,KCl;\,\,III:C{H_3}{(C{H_2})_{11}}OSO_3^ - N{a^ + }$

Answer
564.9k+ views
Hint:The surface tension of a liquid is linked with the magnitude of their intermolecular forces present in the liquid.
Complete step by step solution:
(I) In the figure we can see that the surface tension of aqueous solution is decreasing as the concentration increases.
When $C{H_3}OH$ is mixed with ${H_2}O$ to form an aqueous solution of $C{H_3}OH$, the hydrogen bond between the water molecules present is broken and there is a decrease in the force of attraction. So when there is a decrease in the force of attraction between the molecules the surface tension will also decrease. That is why the aqueous solution of $C{H_3}OH$ decreases its surface tension when concentration is increased.
(II) In the figure we can see that the surface tension of aqueous solution is increasing when the concentration is increasing.
When we add $KCl$ to water there is a heat of hydration. That means ${K^ + }$ and $C{l^ - }$ get solvated by water molecules. When this happens, there is an increase in the electrostatic force of attraction. So, when there is an increase in the force of attraction the surface tension also increases. That Is why the aqueous solution of $KCl$ increases its surface tension when the concentration is increased.
(III) In the figure we can see that there is a sharp decrease in surface tension when concentration is increased.
When we add $C{H_3}{(C{H_2})_{11}}OS{O_3}^ - N{a^ + }$ to water the, the surfactant molecules adsorb at the water surface. Now, at the surface some of the water molecules are replaced by the surfactants. The force of attraction between water and surfactants are lesser than the two water molecules. Therefore after some time, we can see a rapid decrease in the surface tension of the solution
Note:
1. For solving this easier, we could have started with surfactant and matching it with figure (III), as we know that it is a characteristic property of surfactants. And then we can move to $KCl$ and finally to $C{H_3}OH$. This way we could save a lot of time.
2. The surface tension of any pure fluids does not change with the concentration. It will remain the same whatever the concentrations are.
Complete step by step solution:
(I) In the figure we can see that the surface tension of aqueous solution is decreasing as the concentration increases.
When $C{H_3}OH$ is mixed with ${H_2}O$ to form an aqueous solution of $C{H_3}OH$, the hydrogen bond between the water molecules present is broken and there is a decrease in the force of attraction. So when there is a decrease in the force of attraction between the molecules the surface tension will also decrease. That is why the aqueous solution of $C{H_3}OH$ decreases its surface tension when concentration is increased.
(II) In the figure we can see that the surface tension of aqueous solution is increasing when the concentration is increasing.
When we add $KCl$ to water there is a heat of hydration. That means ${K^ + }$ and $C{l^ - }$ get solvated by water molecules. When this happens, there is an increase in the electrostatic force of attraction. So, when there is an increase in the force of attraction the surface tension also increases. That Is why the aqueous solution of $KCl$ increases its surface tension when the concentration is increased.
(III) In the figure we can see that there is a sharp decrease in surface tension when concentration is increased.
When we add $C{H_3}{(C{H_2})_{11}}OS{O_3}^ - N{a^ + }$ to water the, the surfactant molecules adsorb at the water surface. Now, at the surface some of the water molecules are replaced by the surfactants. The force of attraction between water and surfactants are lesser than the two water molecules. Therefore after some time, we can see a rapid decrease in the surface tension of the solution
Note:
1. For solving this easier, we could have started with surfactant and matching it with figure (III), as we know that it is a characteristic property of surfactants. And then we can move to $KCl$ and finally to $C{H_3}OH$. This way we could save a lot of time.
2. The surface tension of any pure fluids does not change with the concentration. It will remain the same whatever the concentrations are.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




