
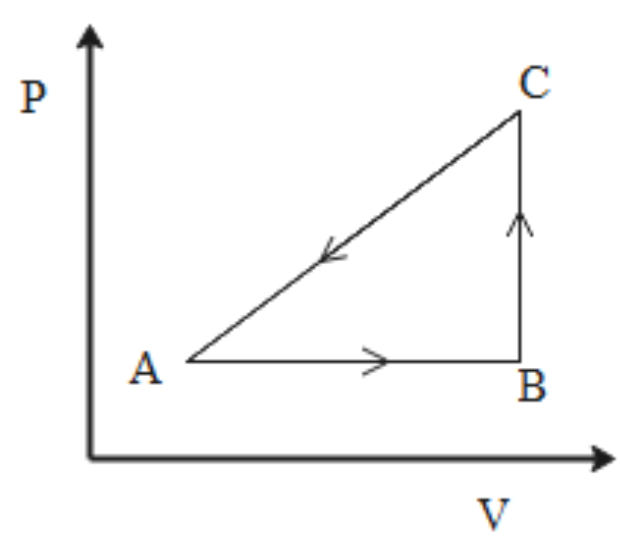
The P-V diagram of the system undergoing thermodynamic transformation is shown in the figure. The work done by the system is going from $A \to B \to C$ is 50 J and 20 cal heat is given to the system. The change in the internal energy between A and C is
A. 34 J
B. 70 J
C. 84 J
D. 134 J

Answer
585.3k+ views
Hint: We are given the amount of energy given to the system and the work done by the system. The first law of thermodynamics gives the relation that the amount of heat energy absorbed by a system is equal to the sum of the change in the internal energy of the system and the amount of work done by the system.
Formula used:
The first law of thermodynamics has the following expression:
\[\Delta Q = \Delta U + \Delta W\]
Here dQ represents the amount of heat energy absorbed by the system, dU represents the change in the internal energy of the system upon absorbing the heat dQ while dW denotes the amount of work done by the system.
Also, we require the following relation:
$1cal = 4.18J$
Complete step-by-step solution:
We have studied the first law of thermodynamics which states that when a system absorbs a certain amount of heat energy then one part of this energy is used to change the internal energy of the system while the rest of heat energy is utilized in doing work on the system.
Mathematically, it can be represented by the following expression.
\[\Delta Q = \Delta U + \Delta W\]
Here the symbols have their usual meanings.
We are given the amount of energy given to the system and the work done by the system. The values are
$
\Delta Q = 20cal = 20 \times 4.2 = 84J \\
\Delta W = - 50J \\
$
Now using the first law of thermodynamics, we can get the change in internal energy of the system which is given as
$\Delta U = \Delta Q - \Delta W$
Now we will insert the known values of amount of heat energy absorbed and amount of work done. Doing so, we get
$\Delta U = 84 - \left( { - 50} \right) = 84 + 50 = 134J$
Hence, the correct answer is option D.
Note: 1. When work is done on a thermodynamic system then it is taken to be negative but if work is done by the system, then it is taken as positive.
2. The work done in a thermodynamic system is equal to the area covered in the PV diagram.
Formula used:
The first law of thermodynamics has the following expression:
\[\Delta Q = \Delta U + \Delta W\]
Here dQ represents the amount of heat energy absorbed by the system, dU represents the change in the internal energy of the system upon absorbing the heat dQ while dW denotes the amount of work done by the system.
Also, we require the following relation:
$1cal = 4.18J$
Complete step-by-step solution:
We have studied the first law of thermodynamics which states that when a system absorbs a certain amount of heat energy then one part of this energy is used to change the internal energy of the system while the rest of heat energy is utilized in doing work on the system.
Mathematically, it can be represented by the following expression.
\[\Delta Q = \Delta U + \Delta W\]
Here the symbols have their usual meanings.
We are given the amount of energy given to the system and the work done by the system. The values are
$
\Delta Q = 20cal = 20 \times 4.2 = 84J \\
\Delta W = - 50J \\
$
Now using the first law of thermodynamics, we can get the change in internal energy of the system which is given as
$\Delta U = \Delta Q - \Delta W$
Now we will insert the known values of amount of heat energy absorbed and amount of work done. Doing so, we get
$\Delta U = 84 - \left( { - 50} \right) = 84 + 50 = 134J$
Hence, the correct answer is option D.
Note: 1. When work is done on a thermodynamic system then it is taken to be negative but if work is done by the system, then it is taken as positive.
2. The work done in a thermodynamic system is equal to the area covered in the PV diagram.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




