
the product $ Z $ of the following reaction is:
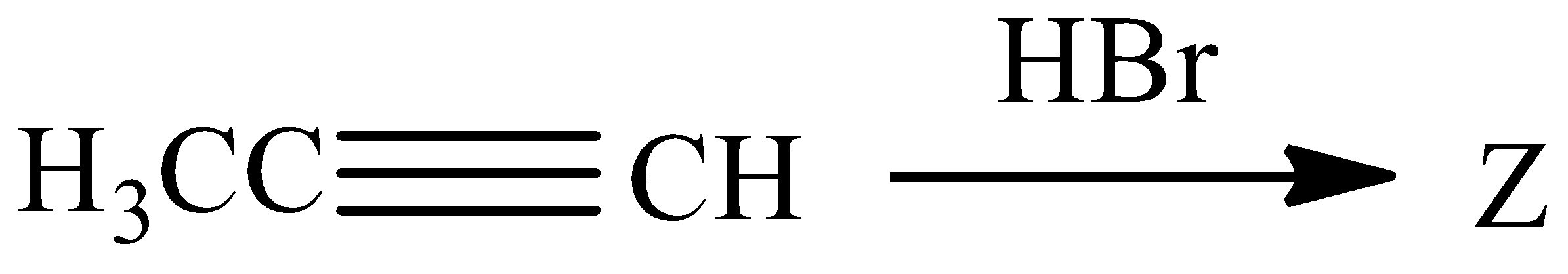
$ A){H_3}CC{H_2}CHB{r_2} $
$ B){H_3}CCHBrC{H_2}Br $
$ C){H_3}CCB{r_2}C{H_3} $
$ D)BrC{H_2}C{H_2}Br $
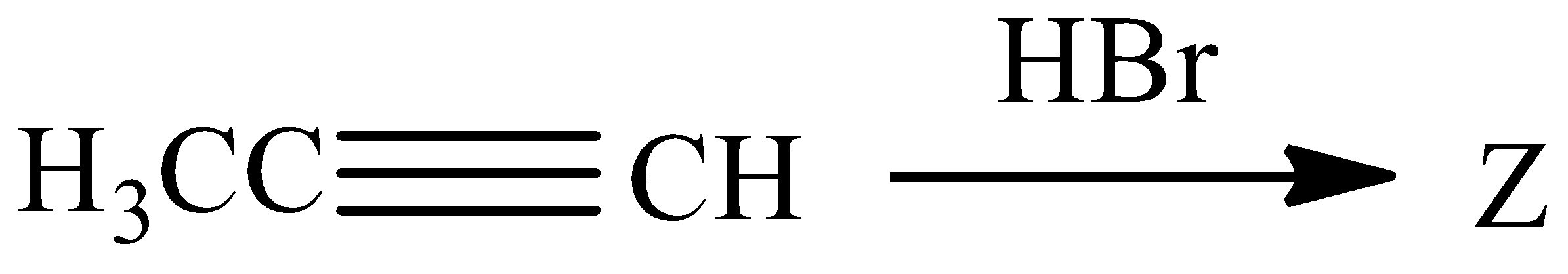
Answer
516.6k+ views
Hint: This is an electrophilic substitution reaction. In this alkenes react with hydrogen bromide in the cold. The double bond breaks and a hydrogen atom ends up attached to one of the carbons and a bromine atom to the other. Symmetrical alkenes are dealt with first. These are alkenes where identical groups are attached to each end of the carbon-carbon double bond.
Complete answer:
Hydrogen bromide adds to alkenes to create alkyl halides. In this the reaction is that the pi bond of the alkene acts as a weak nucleophile and reacts with the electrophilic proton of $ HBr $ . In the first step of the reaction as the protonation of the pi bond. A carbocation intermediate is formed along with the bromide anion during the initial step of the reaction.
In unsymmetrical alkenes, the more stable of the two possible carbocations will form predominantly. Carbocations are stabilized by either the presence of alkyl groups, adjacent pi bonds, or atoms with a lone pair attached to the cationic carbon atom.
In this reaction, the propyne reacts with hydrogen bromide and forms $ 2,2 - $ dibromopropane.
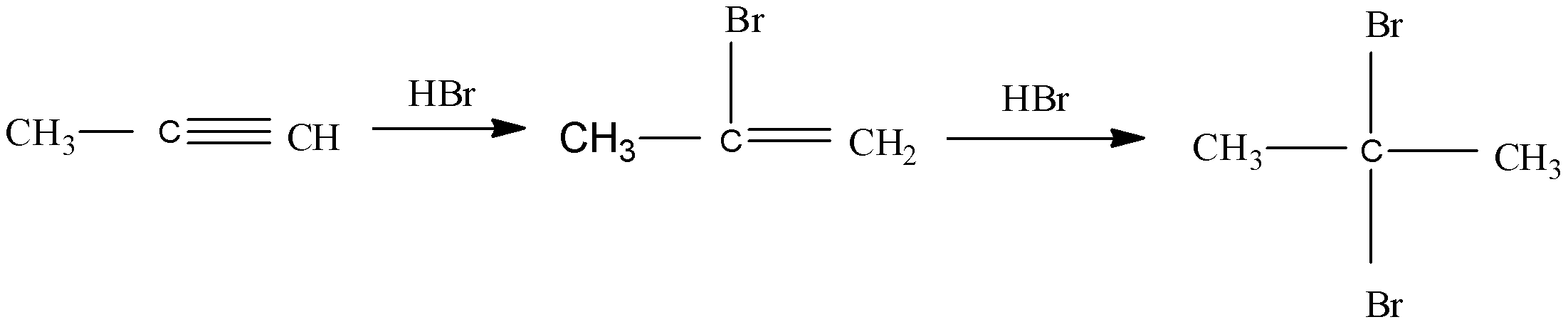
So, the correct answer is $ (C){H_3}CCB{r_2}C{H_3} $ .
Note:
Hydrogen bromide is highly corrosive, anhydrous appears as a colorless gas with a pungent irritating odor. Heavier than air. Prolonged exposure to fire or intense heat may result in the violent rupture and rocketing of the container and also irritating to inhalation severe irritant to the eyes, skin, and nasal passages high concentrations may penetrate to the lungs resulting in edema and hemorrhage
Complete answer:
Hydrogen bromide adds to alkenes to create alkyl halides. In this the reaction is that the pi bond of the alkene acts as a weak nucleophile and reacts with the electrophilic proton of $ HBr $ . In the first step of the reaction as the protonation of the pi bond. A carbocation intermediate is formed along with the bromide anion during the initial step of the reaction.
In unsymmetrical alkenes, the more stable of the two possible carbocations will form predominantly. Carbocations are stabilized by either the presence of alkyl groups, adjacent pi bonds, or atoms with a lone pair attached to the cationic carbon atom.
In this reaction, the propyne reacts with hydrogen bromide and forms $ 2,2 - $ dibromopropane.

So, the correct answer is $ (C){H_3}CCB{r_2}C{H_3} $ .
Note:
Hydrogen bromide is highly corrosive, anhydrous appears as a colorless gas with a pungent irritating odor. Heavier than air. Prolonged exposure to fire or intense heat may result in the violent rupture and rocketing of the container and also irritating to inhalation severe irritant to the eyes, skin, and nasal passages high concentrations may penetrate to the lungs resulting in edema and hemorrhage
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




