
What will be the product (X) of the reaction?
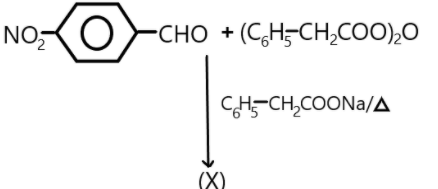


Answer
570k+ views
Hint:As we know that when an aromatic aldehyde reacts with an aliphatic carboxylic acid anhydride in the presence of salt of that carboxylic acid it results into aldol type condensation and results in the formation of an unsaturated acid. This is basically the Perkin’s condensation reaction.
Complete answer:
As we are well aware with the Perkin’s condensation reaction which involves a type of aldol condensation where an aromatic aldehyde reacts with an aliphatic carboxylic acid anhydride and forms an α, β-unsaturated acid in the presence of sodium or potassium salt of the carboxylic acid. The alkali salt of carboxylic acid acts a base catalyst in this reaction.
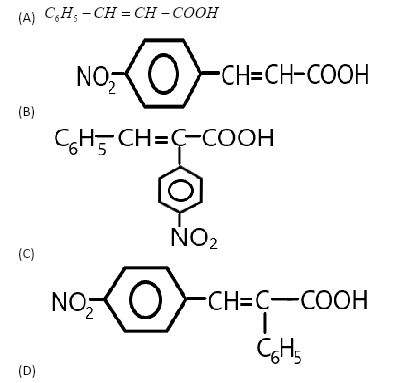
In the given reaction, when \[4\]-Nitrobenzaldehyde will react with the phenylacetic acid anhydride in the presence of sodium salt of phenylacetic acid anhydride, it will be condensed and results in the formation of \[4\]- nitro-$1 - $ene- $2$-phenyl acetic acid which is the fourth option here. We can show the whole reaction as:
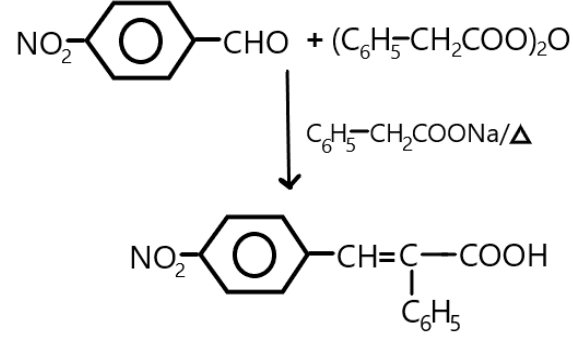
Additional information: Nitrobenzaldehyde is a crystalline powder which has yellow to brown colour and is an odourless compound. It is soluble in water, ethanol and benzene but is air sensitive. It is also an irritant. Perkin’s reaction was first used to produce cinnamic acids.
Note:Sodium salts of carboxylic acids are preferred over potassium salts because under normal conditions sodium salts give a good amount of yield of products whereas potassium under the same conditions produces less product.
Complete answer:
As we are well aware with the Perkin’s condensation reaction which involves a type of aldol condensation where an aromatic aldehyde reacts with an aliphatic carboxylic acid anhydride and forms an α, β-unsaturated acid in the presence of sodium or potassium salt of the carboxylic acid. The alkali salt of carboxylic acid acts a base catalyst in this reaction.
In the given reaction, when \[4\]-Nitrobenzaldehyde will react with the phenylacetic acid anhydride in the presence of sodium salt of phenylacetic acid anhydride, it will be condensed and results in the formation of \[4\]- nitro-$1 - $ene- $2$-phenyl acetic acid which is the fourth option here. We can show the whole reaction as:

Additional information: Nitrobenzaldehyde is a crystalline powder which has yellow to brown colour and is an odourless compound. It is soluble in water, ethanol and benzene but is air sensitive. It is also an irritant. Perkin’s reaction was first used to produce cinnamic acids.
Note:Sodium salts of carboxylic acids are preferred over potassium salts because under normal conditions sodium salts give a good amount of yield of products whereas potassium under the same conditions produces less product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




