
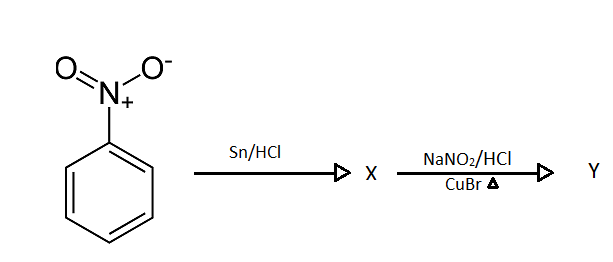
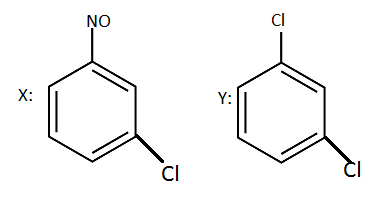
The product $X$ and $Y$ in the following reaction sequences are:
A.
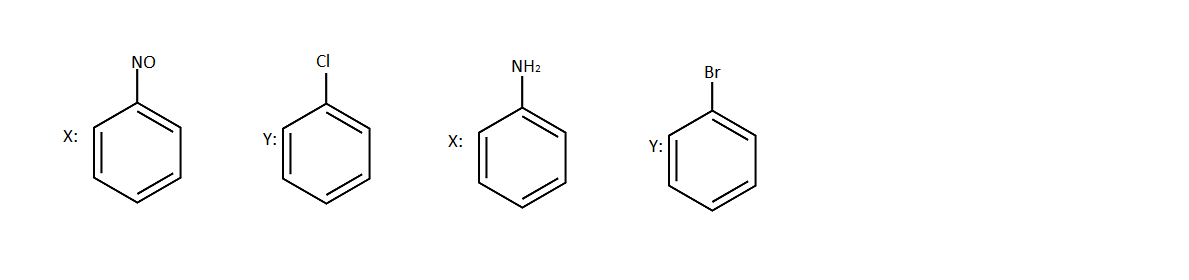
B.
C.
D.





Answer
587.4k+ views
Hint: In organic reactions the work of $Sn/HCl$ , tin in hydrochloric acid is to reduce all the functional groups present in it and \[NaN{O_2}/HCl\] is a reagent responsible for diazotization reaction means adding the two moles of a compound through the $ - N = N - $ bond.
Complete step by step answer:
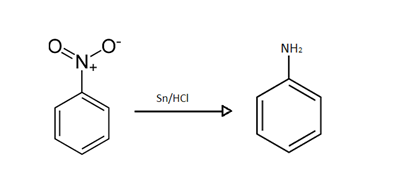
As we can see that $Sn/HCl$ is added to Nitrobenzene in the starting of a sequential reaction.
The work of $Sn/HCl$ in this reaction is to simply reduce it to its lowest form.
So, $Sn/HCl$ will reduce nitrobenzene to aniline and our obtained product $X$ will be aniline.
We can also understand this diagrammatically as following:
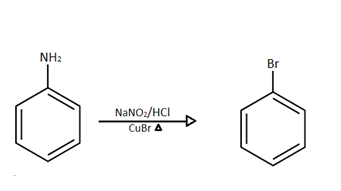
Further we can see that \[NaN{O_2}/HCl\] and $CuBr$ are introduced with heat.
So our aniline which is produced in the previous step will react with these reagents.
Firstly diazotization reaction will occur due to the presence of \[NaN{O_2}/HCl\] reagent and further when it reacts with $CuBr$ and it gives bromobenzene as a product.
We can also understand this diagrammatically as following:
Hence the overall occurrence of sequential reaction is:
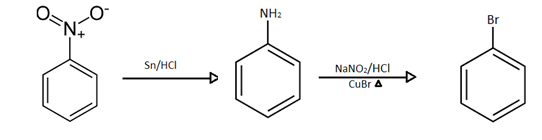
And our products are:
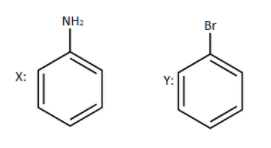
So option (B) is correct.
Note:
The basic method to solve the sequential reactions is that we should picturise the reactions in our mind and start solving them one by one. Every product of the reaction is reactant of some other reaction so if we find any one product wrong the whole sequence will damage.
Complete step by step answer:
As we can see that $Sn/HCl$ is added to Nitrobenzene in the starting of a sequential reaction.
The work of $Sn/HCl$ in this reaction is to simply reduce it to its lowest form.
So, $Sn/HCl$ will reduce nitrobenzene to aniline and our obtained product $X$ will be aniline.
We can also understand this diagrammatically as following:

Further we can see that \[NaN{O_2}/HCl\] and $CuBr$ are introduced with heat.
So our aniline which is produced in the previous step will react with these reagents.
Firstly diazotization reaction will occur due to the presence of \[NaN{O_2}/HCl\] reagent and further when it reacts with $CuBr$ and it gives bromobenzene as a product.
We can also understand this diagrammatically as following:

Hence the overall occurrence of sequential reaction is:

And our products are:

So option (B) is correct.
Note:
The basic method to solve the sequential reactions is that we should picturise the reactions in our mind and start solving them one by one. Every product of the reaction is reactant of some other reaction so if we find any one product wrong the whole sequence will damage.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




