
What would be the product ratio $\dfrac{x}{y}$ in the chlorination of propane if all the hydrogen were abstracted at equal rate?
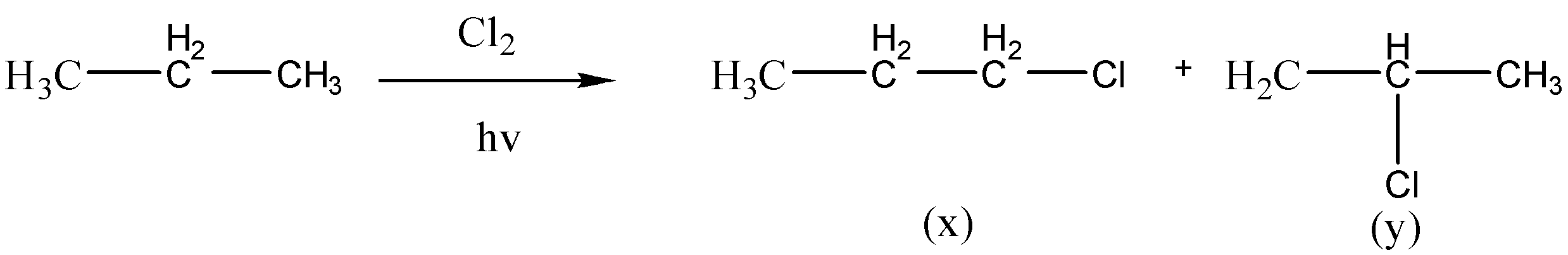
A.$\dfrac{1}{3}$
B.$\dfrac{3}{1}$
C.$\dfrac{9}{1}$
D.$\dfrac{1}{9}$
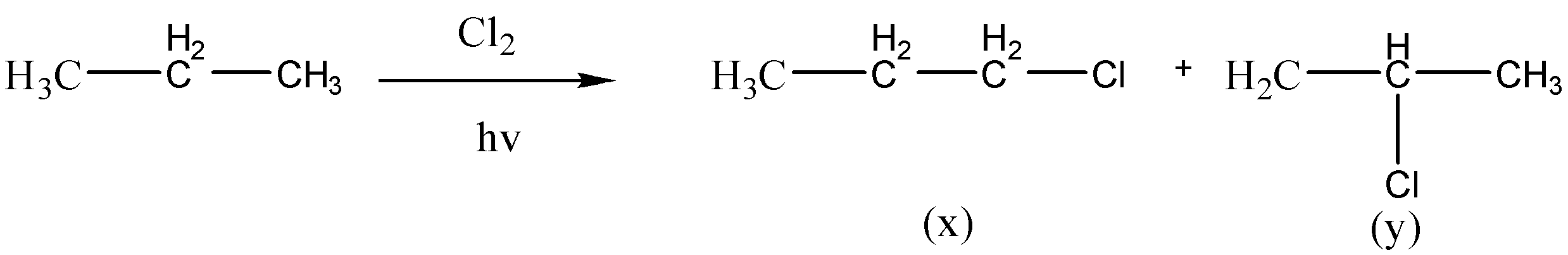
Answer
514.5k+ views
Hint: Here, we are asked about the product ratio which tells us how much reactant has reacted with the other reactant giving product afterwards. In order to know that we should know which one of the products is more stable because more is the stability, more will be the product formed.
Complete answer: So, here we are given a reaction in which propane is undergoing chlorination which is further giving us two products, that is, $1 - chloropropane$ $(x)$ and $2 - chloropropane$ $(y)$, now from these two product we need to find out the product ratio between both of the products which are formed. And we can figure this out by looking at their mechanism, that is, which carbocation is forming and by looking at their rate of reactivity.
So, we know that , there are three types of carbocation which can be formed, that is, tertiary$(3^\circ )$, secondary$(2^\circ )$ and primary$(1^\circ )$. So, let’s have a look at their order of reactivity:
$3^\circ > 2^\circ > 1^\circ $, this means that tertiary carbocation is more reactive as compared to primary carbocation.
Now, chlorination is a form of halogenation, which means that in halogenation reactions, the halogen molecule replaces the hydrogen atom, so in chlorination reactions, chlorine replaces hydrogen atom in the reaction. Now, let’s have a look at the rate at which chlorine replaces hydrogen atoms from the compounds:
$3^\circ $carbocation replaces hydrogen with the rate of $5$, $2^\circ$ carbocation replaces hydrogen with the rate of $3.8$ and $1^\circ $ carbocation replaces hydrogen with the rate of $1$.
Since, $x$is a primary carbocation = $6 \times 1 = 6$
And $y$ is a tertiary carbocation = $2 \times 3.8 = 6.4$
Let’s find out the ratio of $x$and $y$= $\dfrac{x}{y} = \dfrac{{6.4}}{6} = \dfrac{{3.2}}{{3.0}}$
But it is given in the question that the rate is equal, therefore-
$
x = 6 \times 1 = 6 \\
y = 2 \times 1 = 2 \\
$
Now, let’s find out the product ratio $ = \dfrac{6}{2} = \dfrac{3}{1}$
Therefore, the correct option is- Option B.
Note:
Halogenation is the reaction of replacement of hydrogen atoms with the halogen atoms. Halogens are present in groups- $17$ such as Fluorine $(F)$, Chlorine $(Cl)$, Bromine $(Br)$, iodine $(I)$, etc. Halogens have seven electrons in their outermost shell. And they are known to be the electronegative elements and the electronegativity of the elements in the group decreases down the group due to the increase in the size of the atom.
Complete answer: So, here we are given a reaction in which propane is undergoing chlorination which is further giving us two products, that is, $1 - chloropropane$ $(x)$ and $2 - chloropropane$ $(y)$, now from these two product we need to find out the product ratio between both of the products which are formed. And we can figure this out by looking at their mechanism, that is, which carbocation is forming and by looking at their rate of reactivity.
So, we know that , there are three types of carbocation which can be formed, that is, tertiary$(3^\circ )$, secondary$(2^\circ )$ and primary$(1^\circ )$. So, let’s have a look at their order of reactivity:
$3^\circ > 2^\circ > 1^\circ $, this means that tertiary carbocation is more reactive as compared to primary carbocation.
Now, chlorination is a form of halogenation, which means that in halogenation reactions, the halogen molecule replaces the hydrogen atom, so in chlorination reactions, chlorine replaces hydrogen atom in the reaction. Now, let’s have a look at the rate at which chlorine replaces hydrogen atoms from the compounds:
$3^\circ $carbocation replaces hydrogen with the rate of $5$, $2^\circ$ carbocation replaces hydrogen with the rate of $3.8$ and $1^\circ $ carbocation replaces hydrogen with the rate of $1$.
Since, $x$is a primary carbocation = $6 \times 1 = 6$
And $y$ is a tertiary carbocation = $2 \times 3.8 = 6.4$
Let’s find out the ratio of $x$and $y$= $\dfrac{x}{y} = \dfrac{{6.4}}{6} = \dfrac{{3.2}}{{3.0}}$
But it is given in the question that the rate is equal, therefore-
$
x = 6 \times 1 = 6 \\
y = 2 \times 1 = 2 \\
$
Now, let’s find out the product ratio $ = \dfrac{6}{2} = \dfrac{3}{1}$
Therefore, the correct option is- Option B.
Note:
Halogenation is the reaction of replacement of hydrogen atoms with the halogen atoms. Halogens are present in groups- $17$ such as Fluorine $(F)$, Chlorine $(Cl)$, Bromine $(Br)$, iodine $(I)$, etc. Halogens have seven electrons in their outermost shell. And they are known to be the electronegative elements and the electronegativity of the elements in the group decreases down the group due to the increase in the size of the atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




