
The product formed by the reaction between 2,2,2-trichloroethanal and chlorobenzene in \[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\]is:
A) Chloretone
B) DDT
C) Chlorobenzyl Chloride
D) Benzene sulphonic acid
Answer
559.2k+ views
Hint: Here, the reaction is given between the aldehyde and halobenzene in presence of sulphuric acid. 2,2,2-trichloroethanal is also called chloral, it is a polyhalogen compound containing three chlorine atoms and chlorobenzene is a monohalogen compound containing one chlorine atom.
Complete step-by-step answer:
The reaction between the 2,2,2-trichloroethanal and chlorobenzene in presence of the sulphuric acid is as follows:
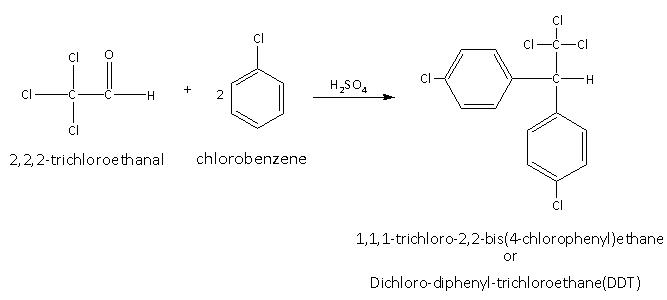
Here, for one mole of the 2,2,2-trichloroethanal two moles chlorobenzene are required to obtain the product DDT.
Here, in the reaction oxygen of the aldehyde and two hydrogens of the benzene molecules are removed as water molecules in presence of the sulphuric acid which is a strong dehydrating agent.
Here, option (A) chloretone is not obtained from the given reactants hence, it is an incorrect answer.
Here, option (B) DDT is the correct answer to the question.
Here, option(C) chlorobenzyl chloride is also an incorrect answer to the given question.
Now, option (D) benzene sulphuric acid is also an incorrect answer to the given question.
Additional Information: The chemical formula of the DDT is \[{{\text{C}}_{{\text{14}}}}{{\text{H}}_{\text{9}}}{\text{C}}{{\text{l}}_{\text{5}}}\].
DDT is most commonly used as an insecticide against houseflies, bugs, other insects but is a non-selective insecticide as it kills good as well as bad insects. Nowadays because of its hazardous effect on human health and the environment it is banned. It is carcinogenic and causes cancer to human beings.
Note: Here, in the reaction, sulphuric acid is used which acts as a dehydrating agent. The dehydrating agent is the substance that removes the water from the reactants in the reaction that is causing the dehydration hence the name is given as dehydrating agent. The different dehydrating agents used in reactions are sulphuric acid, concentrated phosphoric acid, hot aluminium oxide, ethanol, concentrated hydrochloric acid, etc.
Complete step-by-step answer:
The reaction between the 2,2,2-trichloroethanal and chlorobenzene in presence of the sulphuric acid is as follows:

Here, for one mole of the 2,2,2-trichloroethanal two moles chlorobenzene are required to obtain the product DDT.
Here, in the reaction oxygen of the aldehyde and two hydrogens of the benzene molecules are removed as water molecules in presence of the sulphuric acid which is a strong dehydrating agent.
Here, option (A) chloretone is not obtained from the given reactants hence, it is an incorrect answer.
Here, option (B) DDT is the correct answer to the question.
Here, option(C) chlorobenzyl chloride is also an incorrect answer to the given question.
Now, option (D) benzene sulphuric acid is also an incorrect answer to the given question.
Additional Information: The chemical formula of the DDT is \[{{\text{C}}_{{\text{14}}}}{{\text{H}}_{\text{9}}}{\text{C}}{{\text{l}}_{\text{5}}}\].
DDT is most commonly used as an insecticide against houseflies, bugs, other insects but is a non-selective insecticide as it kills good as well as bad insects. Nowadays because of its hazardous effect on human health and the environment it is banned. It is carcinogenic and causes cancer to human beings.
Note: Here, in the reaction, sulphuric acid is used which acts as a dehydrating agent. The dehydrating agent is the substance that removes the water from the reactants in the reaction that is causing the dehydration hence the name is given as dehydrating agent. The different dehydrating agents used in reactions are sulphuric acid, concentrated phosphoric acid, hot aluminium oxide, ethanol, concentrated hydrochloric acid, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




