
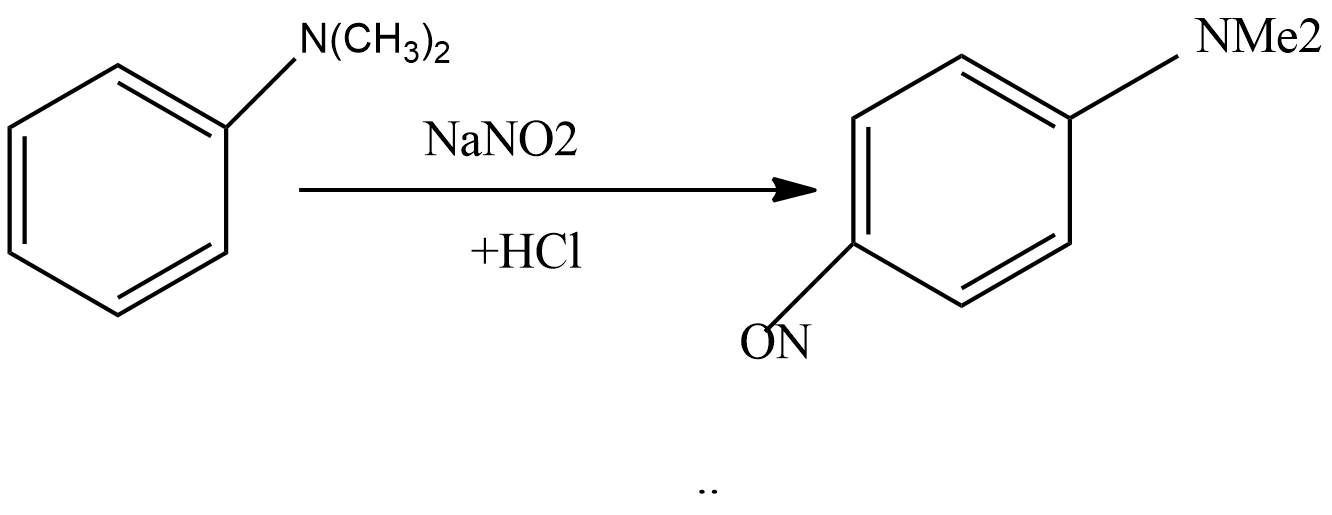
What would be the product for the following reaction?
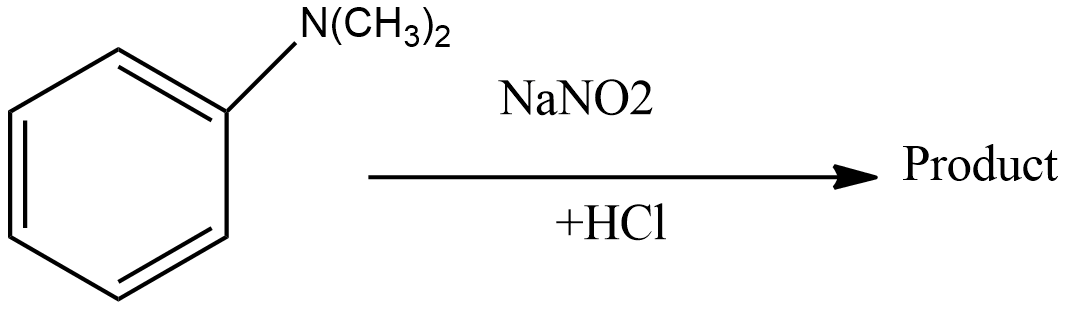
A. $Ph - Cl$
B. $Ph - {N_2}$
C.
D.
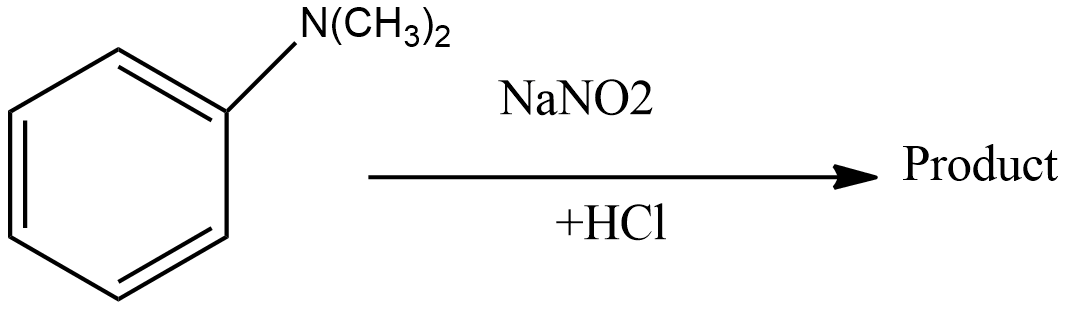


Answer
582.6k+ views
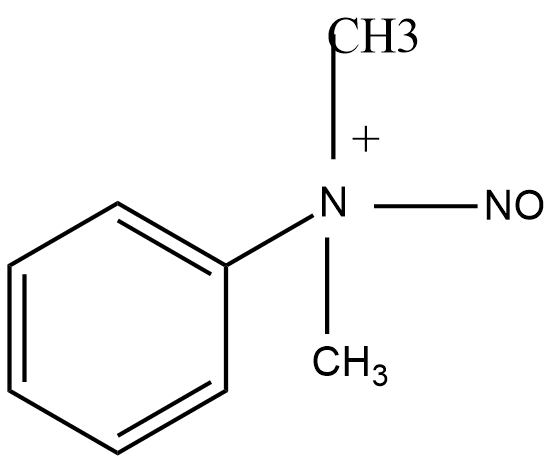
Hint: Since it is tertiary aromatic amines that means there is no N-H bond s nitrosylation on nitrogen and salt formation is not possible. This reaction is used for the distinction of primary, secondary and tertiary amines.
Complete step by step answer: In this reaction, there is electrophilic substitution at the para position. Below are the steps involved in the reaction:
Step1: Sodium nitrite reacts with hydrochloric acid to form sodium chloride and nitrous acid. The reaction for this step is written as:
$
NaN{O_2} + HCl \to NaCl + HN{O_2} \\
\\
$
Step2: This is the protonation step, below is the reaction that shows protonation
$H\mathop O\limits_{..}^{..} N = O + {H^ + } \to {H_2}\mathop O\limits_{..}^ + N = O$
Step 3: This step involves the formation of the nitrosonium ion. Since the oxygen atom is one of the most electronegative atoms, so + charge on it makes it unstable so it withdraws the electrons from the nitrogen atom and forms water and nitrosonium ion $\mathop N\limits^ + = O$.
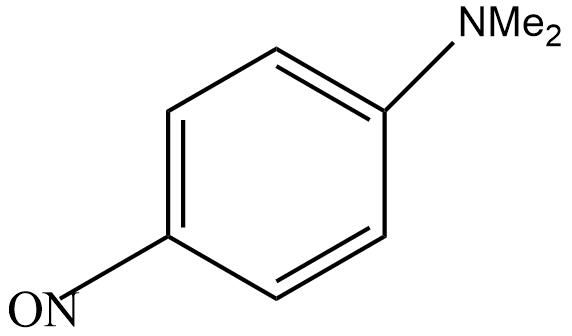
Step 4: In this step, nitrosonium ion attacks on N, N-dimethylaniline to give N, N-dimethyl-p-nitrosoaniline as the main product.
So, the correct answer is “Option C”.
Additional Information: Electrophilic substitution reactions are that chemical reaction in which an electrophile displaces a functional group in a compound. There are mainly two types of electrophilic reaction:
- Electrophilic aromatic substitution reaction
- Electrophilic aliphatic substitution reaction.
- There are some important reactions of Electrophilic aromatic substitution type that take place are aromatic nitration, aromatic halogenation, aromatic sulfonation, and friedel craft reactions.
Note: Here, the dimethylaniline group activates the benzene ring at ortho and para position. But the steric hindrance of the bulkier group does not attack the ortho position. As a result, the compound formed is known as P-nitrosodimethylaniline. It is a dark green crystalline solid and is insoluble in water.
Complete step by step answer: In this reaction, there is electrophilic substitution at the para position. Below are the steps involved in the reaction:
Step1: Sodium nitrite reacts with hydrochloric acid to form sodium chloride and nitrous acid. The reaction for this step is written as:
$
NaN{O_2} + HCl \to NaCl + HN{O_2} \\
\\
$
Step2: This is the protonation step, below is the reaction that shows protonation
$H\mathop O\limits_{..}^{..} N = O + {H^ + } \to {H_2}\mathop O\limits_{..}^ + N = O$
Step 3: This step involves the formation of the nitrosonium ion. Since the oxygen atom is one of the most electronegative atoms, so + charge on it makes it unstable so it withdraws the electrons from the nitrogen atom and forms water and nitrosonium ion $\mathop N\limits^ + = O$.
Step 4: In this step, nitrosonium ion attacks on N, N-dimethylaniline to give N, N-dimethyl-p-nitrosoaniline as the main product.
So, the correct answer is “Option C”.

Additional Information: Electrophilic substitution reactions are that chemical reaction in which an electrophile displaces a functional group in a compound. There are mainly two types of electrophilic reaction:
- Electrophilic aromatic substitution reaction
- Electrophilic aliphatic substitution reaction.
- There are some important reactions of Electrophilic aromatic substitution type that take place are aromatic nitration, aromatic halogenation, aromatic sulfonation, and friedel craft reactions.
Note: Here, the dimethylaniline group activates the benzene ring at ortho and para position. But the steric hindrance of the bulkier group does not attack the ortho position. As a result, the compound formed is known as P-nitrosodimethylaniline. It is a dark green crystalline solid and is insoluble in water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




