
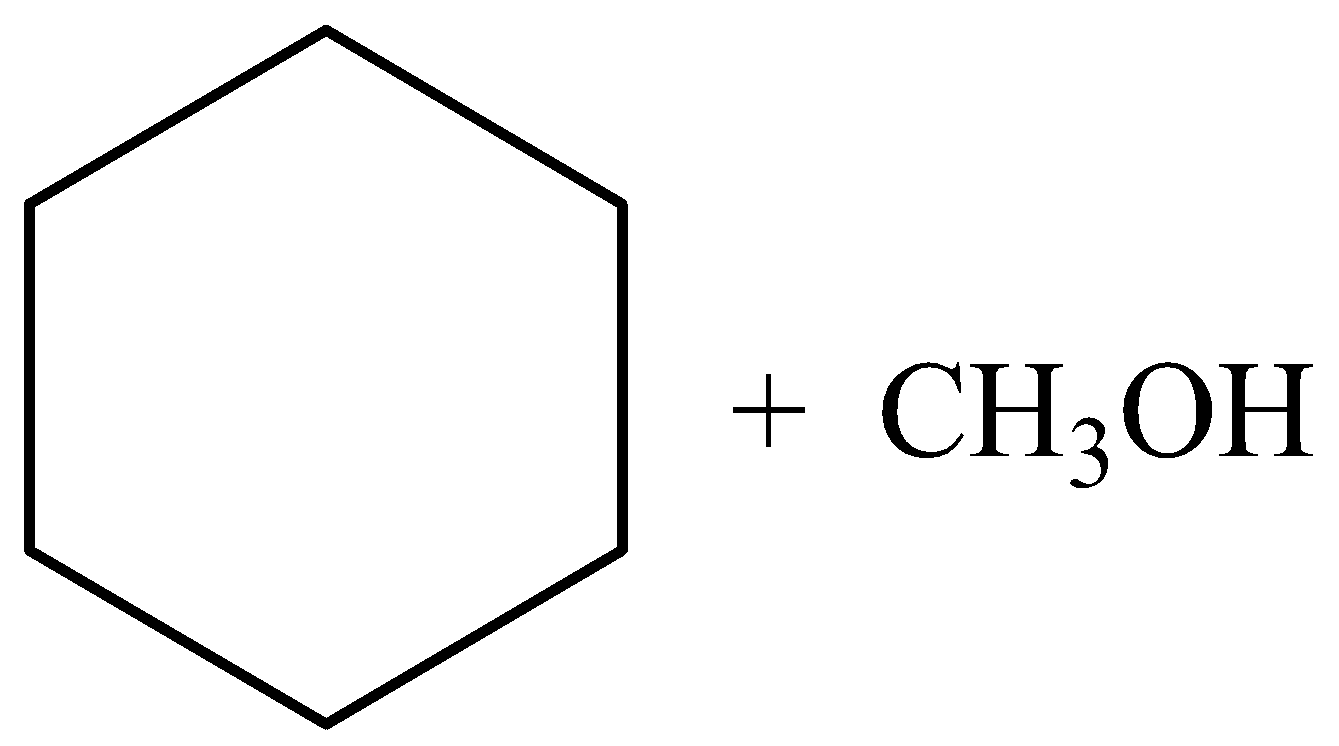
What will be the Product (C) and (D) :
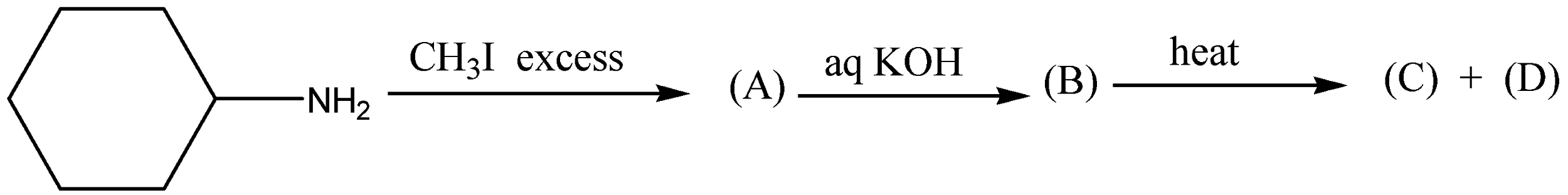
A.
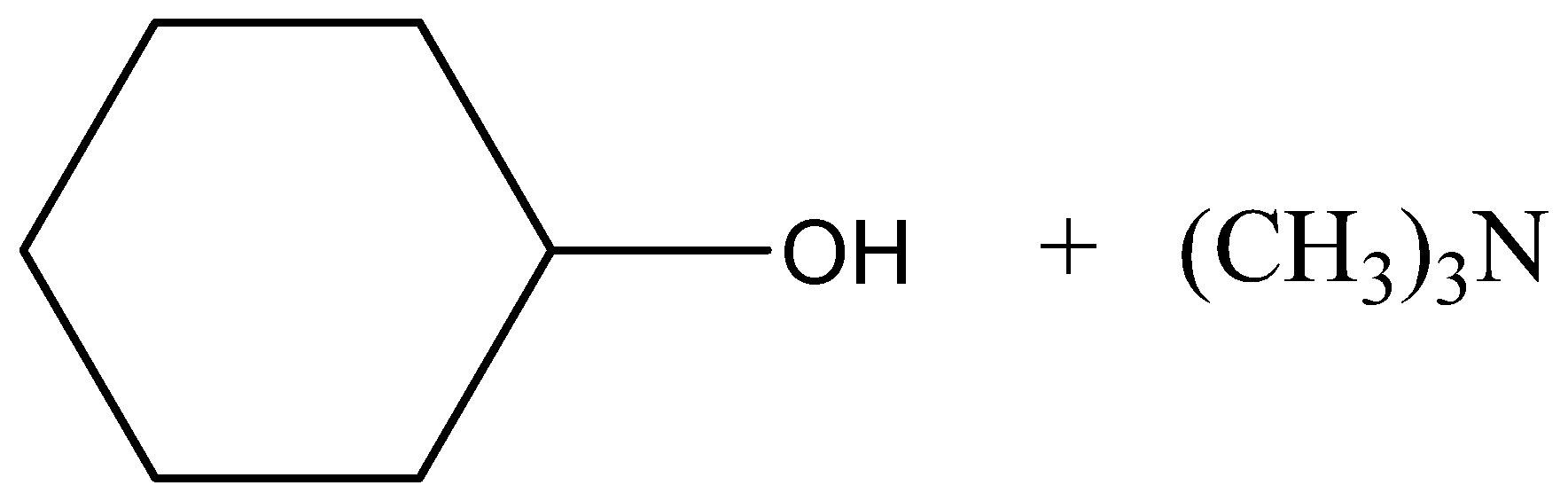
B.
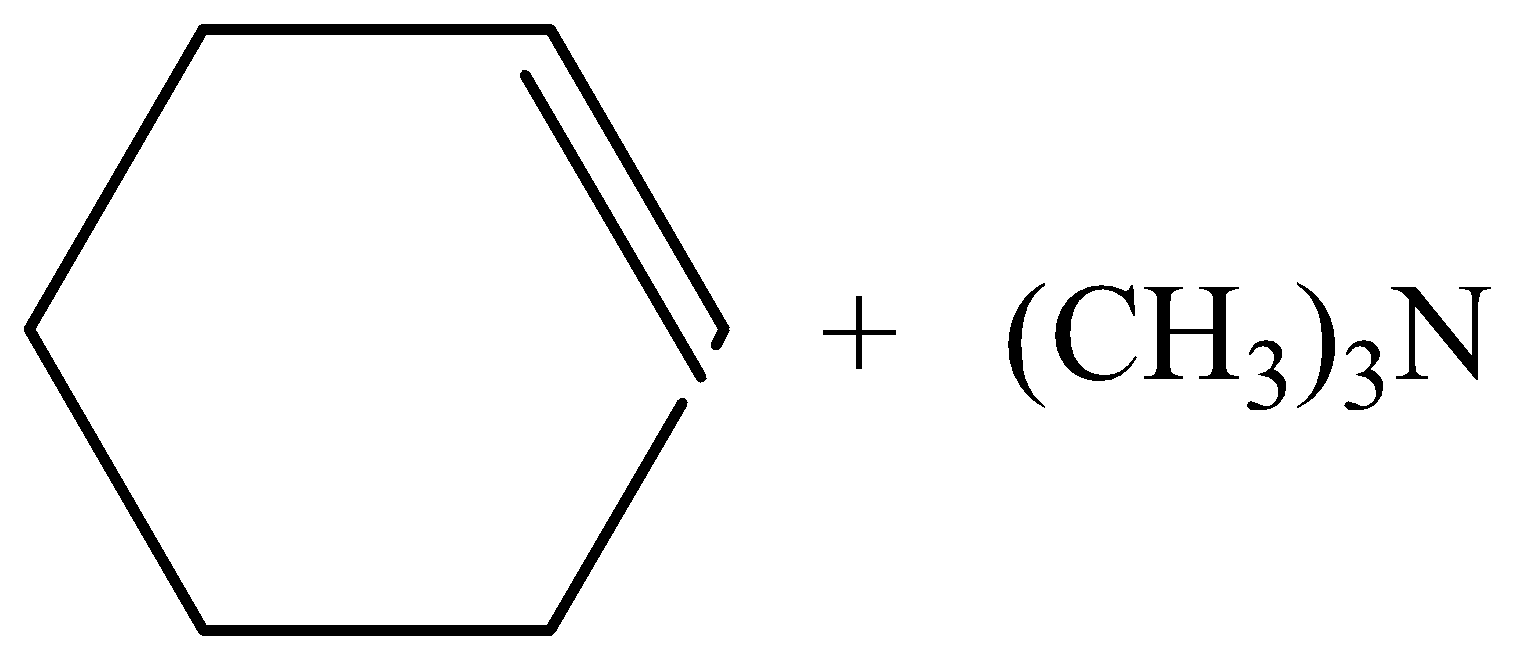
C.
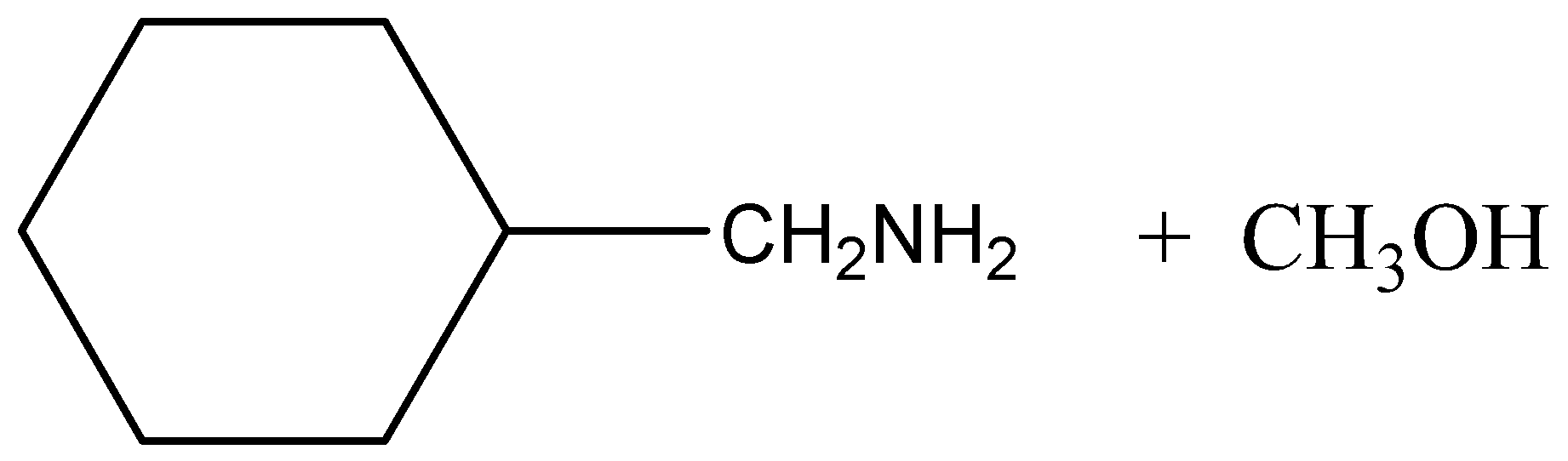
D.
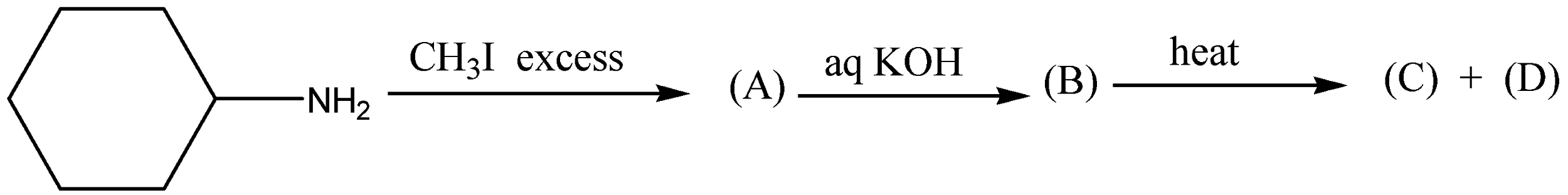




Answer
576.6k+ views
Hint: When aniline and alkyl halide react, a substitution reaction occurs and the halide ion is replaced. When a quaternary ammonium salt reacts with aq. KOH, the elimination reaction of quaternary ammonium salt is termed as the Hofmann Eliminations.
Complete step by step answer:
Aniline is nucleophilic and alkyl halide is electrophilic in nature. When both of these react, a substitution reaction occurs and the halide ion is replaced. With excess of alkyl halide, quaternary ammonium salt formation occurs.
With excess methyl iodide, in the presence of \[N{a_2}C{O_3}\] solution, N, N-dimethylaniline produces N, N, N-trimethylanilinium Iodide
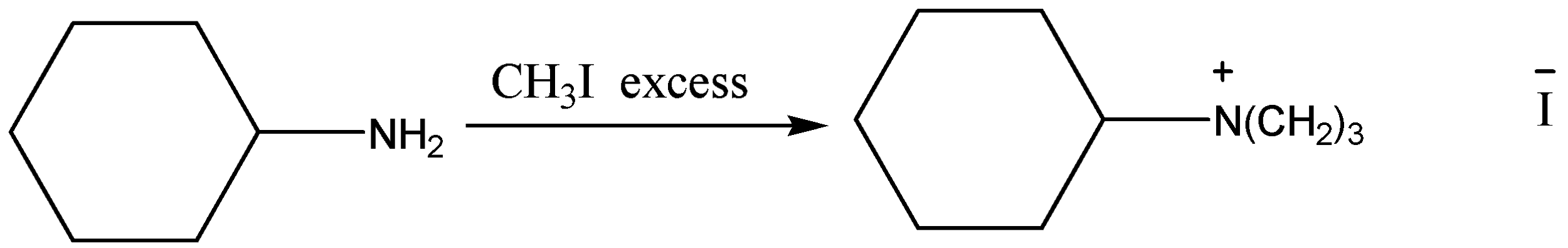
The nitrogen atom of primary amines contains a lone pair of electrons. So, primary amines are strongly nucleophilic in nature. Moreover, the hydrogen atoms on nitrogen are easily replaceable. So, primary amines can act as nucleophiles and can react with alkyl halides. In the presence of excess alkyl halides, one amine molecule can repeatedly react with a number of alkyl halide molecules and result in the formation of a quaternary ammonium salt.
When a quaternary ammonium salt reacts with aq. KOH, the elimination reaction of quaternary ammonium salt is termed as the Hofmann Eliminations. The counter anion of quaternary ammonium salt is often replaced by the more basic hydroxide ion through reaction with KOH (potassium hydroxide). The resulting hydroxide salt must be then heated to effect the E2 like elimination of a three-degree amine.
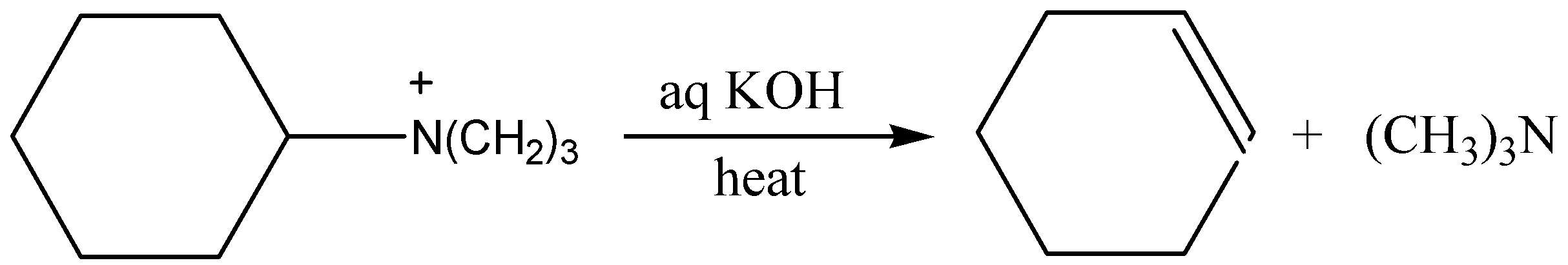
Therefore, the correct answer is option (B).
Note: The less stable trans-cyclohexane is the chief product accompanied by the sis-isomer. An anti E2 transition state would necessarily give the cis-cycloalkane, so the trans isomer must be generated by a syn-elimination.
Complete step by step answer:
Aniline is nucleophilic and alkyl halide is electrophilic in nature. When both of these react, a substitution reaction occurs and the halide ion is replaced. With excess of alkyl halide, quaternary ammonium salt formation occurs.
With excess methyl iodide, in the presence of \[N{a_2}C{O_3}\] solution, N, N-dimethylaniline produces N, N, N-trimethylanilinium Iodide

The nitrogen atom of primary amines contains a lone pair of electrons. So, primary amines are strongly nucleophilic in nature. Moreover, the hydrogen atoms on nitrogen are easily replaceable. So, primary amines can act as nucleophiles and can react with alkyl halides. In the presence of excess alkyl halides, one amine molecule can repeatedly react with a number of alkyl halide molecules and result in the formation of a quaternary ammonium salt.
When a quaternary ammonium salt reacts with aq. KOH, the elimination reaction of quaternary ammonium salt is termed as the Hofmann Eliminations. The counter anion of quaternary ammonium salt is often replaced by the more basic hydroxide ion through reaction with KOH (potassium hydroxide). The resulting hydroxide salt must be then heated to effect the E2 like elimination of a three-degree amine.

Therefore, the correct answer is option (B).
Note: The less stable trans-cyclohexane is the chief product accompanied by the sis-isomer. An anti E2 transition state would necessarily give the cis-cycloalkane, so the trans isomer must be generated by a syn-elimination.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




