
The process of making soap by the hydrolysis of fats and oils with alkalis is called:
A.Hydrolysis
B.Saponification
C.Esterification
D.None of the above.
Answer
573.9k+ views
Hint: Hydrolysis is the chemical process in which a water molecule is added into the substance. In some reactions, the substance and water molecule split and the target substance gains hydronium ion.
Complete step by step answer:
Saponification-as the word suggests, it is the process of formation of soaps by converting fats, oils, lipids in the presence of alkalis( like aqueous\[NaOH\] by the action of heat. For example, sodium palmitate. Thus, soaps are salts of fatty acids with long carbon chains (minimum10).
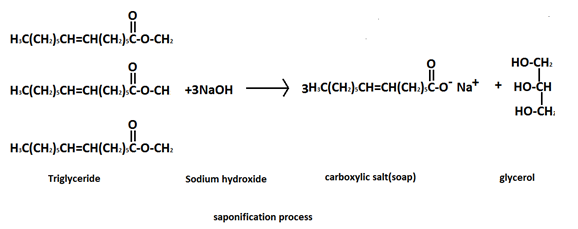
.
Esterification-Alcohol Glycerol and fatty acids condense to form esters. Fats and oils are naturally occurring esters.
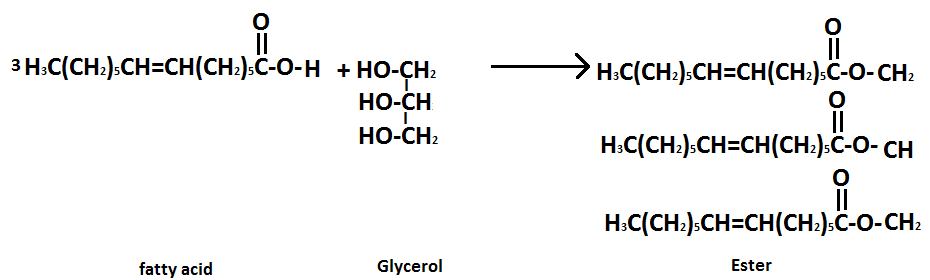
Thus, Saponification, optionB. is the correct answer to the given question.
Note:
\[NaOH\] is one of the best examples of alkalis available, and is commonly called as caustic soda or lye.\[NaOH\], Sodium hydroxide is basic in nature and the most important ingredient for soap making but it is absent from the final product.
Complete step by step answer:
Saponification-as the word suggests, it is the process of formation of soaps by converting fats, oils, lipids in the presence of alkalis( like aqueous\[NaOH\] by the action of heat. For example, sodium palmitate. Thus, soaps are salts of fatty acids with long carbon chains (minimum10).

.
Esterification-Alcohol Glycerol and fatty acids condense to form esters. Fats and oils are naturally occurring esters.

Thus, Saponification, optionB. is the correct answer to the given question.
Note:
\[NaOH\] is one of the best examples of alkalis available, and is commonly called as caustic soda or lye.\[NaOH\], Sodium hydroxide is basic in nature and the most important ingredient for soap making but it is absent from the final product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




