
The principal product of the reaction between methyl butanoate and 2 mole of $C{H_3}MgBr$ after hydrolysis is:
A. ${C_3}{H_7}COC{H_3}$
B. ${C_3}{H_7}C(OH){(C{H_3})_2}$
C. ${C_3}{H_7}CHOC{H_3}$
D. ${C_3}{H_7}COCO{(C{H_3})_2}$
Answer
567.6k+ views
Hint: In this reaction ester is converted to tertiary alcohol on reacting with two moles of Grignard reagent and after on hydrolysis. This reaction takes place in three steps, in first step methyl butanoate reacts with one mole of Grignard reagent and in the second step reacts with second mole of Grignard reagent and in the third step hydrolysis takes place.
Complete step by step answer:
In the given reaction, first reaction between methyl butanoate $C{H_3}C{H_2}C{H_2}COOC{H_3}$ with one mole of $C{H_3}MgBr$(Grignard reagent) takes place. The Grignard reagent $CH_3^ - M{g^ + }B{r^ - }$. The negative charge of the bromine ion attack the oxygen atom of methyl butanoate and MgBr gets attached to the oxygen atom, the double bond of C=O of methyl butanoate shift to oxygen to form a positive charge on the carbon and methyl anion of Grignard reagent attack the carbon cation and methyl group gets attached to it. Again the intermediate ${C_3}{H_7}C(C{H_3})OMgBrOC{H_3}$ is reacted with one mole of Grignard reagent $C{H_3}MgBr$. Again the grignard reagent dissociates into $CH_3^ - M{g^ + }B{r^ - }$. The negative charge of the bromine ion attacks the oxygen atom of intermediate and MgBr gets attached to the oxygen atom. The C-O breaks down to form carbon cation and OMgBr is removed. The negative charge to the methyl anion attack the carbon cation and methyl group gets attached to it forming second intermediate ${C_3}{H_7}C(C{H_3})(OMgBr)C{H_3}$. After that the second intermediate on hydrolysis where the hydrogen ion gets attached to the oxygen and remove MgBrOH to form ${C_3}{H_7}C(OH){(C{H_3})_2}$ as a main product.
The reaction of methyl butanoate with 2 mole of $C{H_3}MgBr$ is shown below.
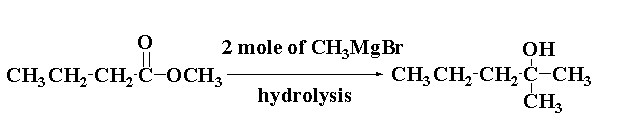
Thus, methyl butanoate $C{H_3}C{H_2}C{H_2}COOC{H_3}$ reacts with 2 mole of Grignard reagent $C{H_3}MgBr$ and on hydrolysis gives 2-methyl-pentan-2-ol ${C_3}{H_7}C(OH){(C{H_3})_2}$.
Therefore, the correct option is B.
Note: The methyl butanoate contains four carbon atoms as a hydrocarbon chain but the product 2-methyl-pentan-2-ol contains five carbon atoms as the hydrocarbon chain. The tertiary alcohol is defined as the alcohol where the hydroxyl group is attached to the carbon atom which is attached to three other carbon atoms.
Complete step by step answer:
In the given reaction, first reaction between methyl butanoate $C{H_3}C{H_2}C{H_2}COOC{H_3}$ with one mole of $C{H_3}MgBr$(Grignard reagent) takes place. The Grignard reagent $CH_3^ - M{g^ + }B{r^ - }$. The negative charge of the bromine ion attack the oxygen atom of methyl butanoate and MgBr gets attached to the oxygen atom, the double bond of C=O of methyl butanoate shift to oxygen to form a positive charge on the carbon and methyl anion of Grignard reagent attack the carbon cation and methyl group gets attached to it. Again the intermediate ${C_3}{H_7}C(C{H_3})OMgBrOC{H_3}$ is reacted with one mole of Grignard reagent $C{H_3}MgBr$. Again the grignard reagent dissociates into $CH_3^ - M{g^ + }B{r^ - }$. The negative charge of the bromine ion attacks the oxygen atom of intermediate and MgBr gets attached to the oxygen atom. The C-O breaks down to form carbon cation and OMgBr is removed. The negative charge to the methyl anion attack the carbon cation and methyl group gets attached to it forming second intermediate ${C_3}{H_7}C(C{H_3})(OMgBr)C{H_3}$. After that the second intermediate on hydrolysis where the hydrogen ion gets attached to the oxygen and remove MgBrOH to form ${C_3}{H_7}C(OH){(C{H_3})_2}$ as a main product.
The reaction of methyl butanoate with 2 mole of $C{H_3}MgBr$ is shown below.

Thus, methyl butanoate $C{H_3}C{H_2}C{H_2}COOC{H_3}$ reacts with 2 mole of Grignard reagent $C{H_3}MgBr$ and on hydrolysis gives 2-methyl-pentan-2-ol ${C_3}{H_7}C(OH){(C{H_3})_2}$.
Therefore, the correct option is B.
Note: The methyl butanoate contains four carbon atoms as a hydrocarbon chain but the product 2-methyl-pentan-2-ol contains five carbon atoms as the hydrocarbon chain. The tertiary alcohol is defined as the alcohol where the hydroxyl group is attached to the carbon atom which is attached to three other carbon atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




