
The prefixes syn- and anti- are used to denote:
A.Structural isomers
B.Conformational isomers
C.Geometrical isomers
D.Optical isomers
Answer
583.5k+ views
Hint: Compounds that have the same molecular formula are called isomers. We are given four types of isomers in the options. Syn and anti sounds like change in the geometry of a molecule by changing the position of substituents. These are similar to cis-trans or $\dfrac{E}{Z}$ types of geometrical isomers we observe in organic molecules.
Complete step-by-step answer:
Geometrical isomers occur when we have restricted rotation somewhere in a molecule such as carbon-carbon double bond or alkenes. They usually differ in their physical properties such as density, polarity, solubility, melting point, boiling point and have different arrangement of substituents.
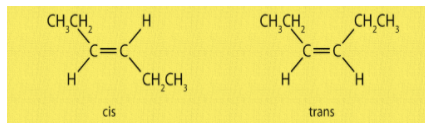
The very common type of geometrical isomers are cis isomers, which have same groups on same side of the double bond of an alkene whereas in trans isomer, same groups lie opposite to each other with respect to double bond. For example,
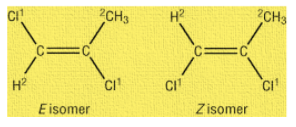
Another type of geometrical isomers is the $\dfrac{E}{Z}$ system. In Z isomer, high priority groups are on the same side of the double bond while in trans the high priority groups are on opposite sides of the double bond. For example,
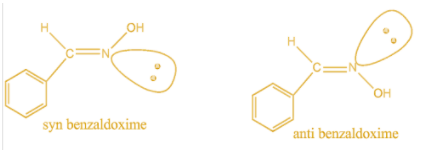
Now comes the \[\dfrac{{syn}}{{anti}}\] type of geometrical isomer which is commonly observed in stereoisomeric oxime. Syn represents the cis position of the hydroxyl group with respect to the hydrogen group whereas in anti, trans position is observed. For example,
Structural isomers are those in which the molecules have the same molecular formula, but their atoms have different arrangements or bonds. For example, Butane and isobutane have the same number of carbon atoms and hydrogen atoms, so their molecular formulas are the same and have different structures.
Isomers which have the same bond connectivity sequence and can be interconverted by rotation around one or more single sigma bonds are known as conformational isomers. For example, cyclohexane has a boat and chair form of conformational isomers.
If the arrangement of atoms in space makes the two isomers non-superimposable mirror images of each other, then we call them optical isomers. These are commonly observed in a glucose molecule.
Hence, the correct option is (C).
Note: Geometrical isomers are not possible in tetrahedral complexes which have two different types of unidentate ligands coordinated with the central metal ion. This is due to its geometry i.e. the relative positions of the unidentate ligands attached to the central metal ion are same with respect to each other. But they are possible for both square planar and octahedral complexes.
Complete step-by-step answer:
Geometrical isomers occur when we have restricted rotation somewhere in a molecule such as carbon-carbon double bond or alkenes. They usually differ in their physical properties such as density, polarity, solubility, melting point, boiling point and have different arrangement of substituents.
The very common type of geometrical isomers are cis isomers, which have same groups on same side of the double bond of an alkene whereas in trans isomer, same groups lie opposite to each other with respect to double bond. For example,

Another type of geometrical isomers is the $\dfrac{E}{Z}$ system. In Z isomer, high priority groups are on the same side of the double bond while in trans the high priority groups are on opposite sides of the double bond. For example,

Now comes the \[\dfrac{{syn}}{{anti}}\] type of geometrical isomer which is commonly observed in stereoisomeric oxime. Syn represents the cis position of the hydroxyl group with respect to the hydrogen group whereas in anti, trans position is observed. For example,

Structural isomers are those in which the molecules have the same molecular formula, but their atoms have different arrangements or bonds. For example, Butane and isobutane have the same number of carbon atoms and hydrogen atoms, so their molecular formulas are the same and have different structures.
Isomers which have the same bond connectivity sequence and can be interconverted by rotation around one or more single sigma bonds are known as conformational isomers. For example, cyclohexane has a boat and chair form of conformational isomers.
If the arrangement of atoms in space makes the two isomers non-superimposable mirror images of each other, then we call them optical isomers. These are commonly observed in a glucose molecule.
Hence, the correct option is (C).
Note: Geometrical isomers are not possible in tetrahedral complexes which have two different types of unidentate ligands coordinated with the central metal ion. This is due to its geometry i.e. the relative positions of the unidentate ligands attached to the central metal ion are same with respect to each other. But they are possible for both square planar and octahedral complexes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




