
The \[pKa\] of acetyl salicylic acid (aspirin) is \[3.5\]. The \[pH\] of gastric juice in the human stomach is about \[2 - 3\] and the \[pH\] in the small intestine is about \[8\]. Aspirin will be:
A.unionised in the small intestine and in the stomach
B.completely ionised in the small intestine and in the stomach
C.ionised in the stomach and almost unionised in the small intestine
D.ionised in the small intestine and almost unionised in the stomach
Answer
586.5k+ views
Hint: Aspirin is weakly acidic in nature. But the dissociation or ionisation of aspirin is more favorable in the basic environment. It will not ionise in an acidic environment as the concentration of hydrogen ions is more.
Complete step by step answer:
Aspirin is a compound which is synthesized using salicylic acid and acetic anhydride or acetyl chloride.
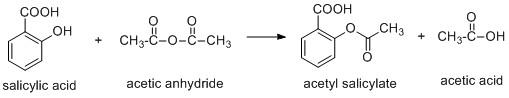
The aspirin is a weak acid and it undergoes ionisation to produce hydrogen ion.
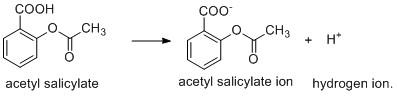
The above ionisation is possible when the products formed i.e. \[{H^ + }\] ion is consumed or removed from the system to generate other products. The \[pH\] of the small intestine is \[8\] which indicates that it is basic in nature. As \[pKa\] of aspirin is greater than the \[pH\] of small intestine, so the former undergoes ionisation in small intestine by combination of $H^+$ and basic \[O{H^ - }\] ion generating water molecule.
The above ionisation is not possible where there is presence of \[{H^ + }\] ion in excess. This can be accounted for by the common ion effect. The common ion effect describes that when a common ion viz. \[{H^ + }\] is already present in the medium such dissociation is unfavorable and it will prevent the forward reaction. As pH in the stomach is \[2 - 3\] which indicates that \[pH > pKa\], so the ionisation will not occur.
Hence the correct option is D. Aspirin will be ionised in the small intestine and almost unionised in the stomach.
Note:
The acidity is the property of acids and is defined by the ability to give hydrogen ions in an acid base reaction. The basicity is the property of bases to accept the hydrogen ion in a reaction.
Complete step by step answer:
Aspirin is a compound which is synthesized using salicylic acid and acetic anhydride or acetyl chloride.

The aspirin is a weak acid and it undergoes ionisation to produce hydrogen ion.

The above ionisation is possible when the products formed i.e. \[{H^ + }\] ion is consumed or removed from the system to generate other products. The \[pH\] of the small intestine is \[8\] which indicates that it is basic in nature. As \[pKa\] of aspirin is greater than the \[pH\] of small intestine, so the former undergoes ionisation in small intestine by combination of $H^+$ and basic \[O{H^ - }\] ion generating water molecule.
The above ionisation is not possible where there is presence of \[{H^ + }\] ion in excess. This can be accounted for by the common ion effect. The common ion effect describes that when a common ion viz. \[{H^ + }\] is already present in the medium such dissociation is unfavorable and it will prevent the forward reaction. As pH in the stomach is \[2 - 3\] which indicates that \[pH > pKa\], so the ionisation will not occur.
Hence the correct option is D. Aspirin will be ionised in the small intestine and almost unionised in the stomach.
Note:
The acidity is the property of acids and is defined by the ability to give hydrogen ions in an acid base reaction. The basicity is the property of bases to accept the hydrogen ion in a reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




