
The peroxide linkage is not present in:
A.) ${H_2}S{O_5}$
B.) $Cr{O_5}$
C.) $HN{O_4}$
D.) $HCl{O_4}$
Answer
574.2k+ views
Hint: The peroxide linkage is that linkage in a compound in which two oxygen atoms are linked together by a single covalent bond. This linkage can be represented as $( - O - O - )$ in a compound. Peroxides are also used for bleaching agents.
Complete step by step answer:
In the peroxide bonds the oxidation state of oxygen is $ - 1$ (instead of normal $ - 2$ for double bonded oxygen). To find out the peroxide bonds in a compound we can consider the oxidation number of a central metal in a compound and if the oxidation number of a central metal in a compound that we are calculating by simple method is coming out to be greater than the maximum possible oxidation state of that central metal then that compound must contain peroxide bonds. These peroxide bonds can compensate the oxidation number of that central metal to a lower value that may be equal to the maximum possible oxidation state of that metal.
To decrease oxidation state by $2$ we need one peroxide bond and to decrease oxidation state by $4$ we need two peroxide bonds.
For option A.), In ${H_2}S{O_5}$, when we calculate the oxidation state of sulfur by simple calculation then it will be:
$
+ 2 + x + ( - 10) = 0 \\
x = + 8 \\
$
But as we know that sulfur has a maximum oxidation state of $ + 6$. So, there will be one peroxide bond in which each oxygen will have $ - 1$ oxidation state instead of $ - 2$. So, it will have one peroxide bond in it. Its structure can be given as:
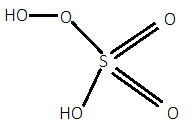
For option B.), In $Cr{O_5}$, when we calculate the oxidation state of chromium by simple calculation then it will be:
$
x + ( - 10) \\
x = + 10 \\
$
But as we know that chromium has a maximum oxidation state of $ + 6$. So, there will be two peroxide bonds in which each oxygen (total four oxygen) will have $ - 1$ oxidation state instead of $ - 2$. So, it will have two peroxide bonds in it. Its structure can be given as:
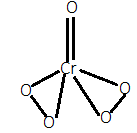
For option C.), In $HN{O_4}$, when we calculate the oxidation state of nitrogen by simple calculation then it will be:
$
+ 1 + x + ( - 8) = 0 \\
x = + 7 \\
$
But as we know that nitrogen has a maximum oxidation state of $ + 5$. So, there will be one peroxide bond in which each oxygen will have $ - 1$ oxidation state instead of $ - 2$. So, it will have one peroxide bond in it. Its structure can be given as:
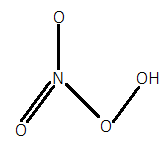
For option D.), In $HCl{O_4}$, when we calculate the oxidation state of chlorine by simple calculation then it will be:
$
+ 1 + x + ( - 8) = 0 \\
x = + 7 \\
$
As we know that chlorine has a maximum oxidation state of $ + 7$. So, there will not be any peroxide bond required. Its structure can be given as:
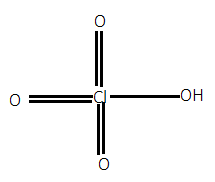
Hence, option D.) is the correct answer.
Note:
Always remember that sulfur has maximum possible oxidation state of $ + 6$, chromium also has $ + 6$ as maximum possible oxidation state, nitrogen has $ + 5$ maximum oxidation state and chlorine has maximum possible oxidation state as $ + 7$.
Complete step by step answer:
In the peroxide bonds the oxidation state of oxygen is $ - 1$ (instead of normal $ - 2$ for double bonded oxygen). To find out the peroxide bonds in a compound we can consider the oxidation number of a central metal in a compound and if the oxidation number of a central metal in a compound that we are calculating by simple method is coming out to be greater than the maximum possible oxidation state of that central metal then that compound must contain peroxide bonds. These peroxide bonds can compensate the oxidation number of that central metal to a lower value that may be equal to the maximum possible oxidation state of that metal.
To decrease oxidation state by $2$ we need one peroxide bond and to decrease oxidation state by $4$ we need two peroxide bonds.
For option A.), In ${H_2}S{O_5}$, when we calculate the oxidation state of sulfur by simple calculation then it will be:
$
+ 2 + x + ( - 10) = 0 \\
x = + 8 \\
$
But as we know that sulfur has a maximum oxidation state of $ + 6$. So, there will be one peroxide bond in which each oxygen will have $ - 1$ oxidation state instead of $ - 2$. So, it will have one peroxide bond in it. Its structure can be given as:

For option B.), In $Cr{O_5}$, when we calculate the oxidation state of chromium by simple calculation then it will be:
$
x + ( - 10) \\
x = + 10 \\
$
But as we know that chromium has a maximum oxidation state of $ + 6$. So, there will be two peroxide bonds in which each oxygen (total four oxygen) will have $ - 1$ oxidation state instead of $ - 2$. So, it will have two peroxide bonds in it. Its structure can be given as:

For option C.), In $HN{O_4}$, when we calculate the oxidation state of nitrogen by simple calculation then it will be:
$
+ 1 + x + ( - 8) = 0 \\
x = + 7 \\
$
But as we know that nitrogen has a maximum oxidation state of $ + 5$. So, there will be one peroxide bond in which each oxygen will have $ - 1$ oxidation state instead of $ - 2$. So, it will have one peroxide bond in it. Its structure can be given as:

For option D.), In $HCl{O_4}$, when we calculate the oxidation state of chlorine by simple calculation then it will be:
$
+ 1 + x + ( - 8) = 0 \\
x = + 7 \\
$
As we know that chlorine has a maximum oxidation state of $ + 7$. So, there will not be any peroxide bond required. Its structure can be given as:

Hence, option D.) is the correct answer.
Note:
Always remember that sulfur has maximum possible oxidation state of $ + 6$, chromium also has $ + 6$ as maximum possible oxidation state, nitrogen has $ + 5$ maximum oxidation state and chlorine has maximum possible oxidation state as $ + 7$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




