
The pair that has similar shape is
A. \[B{F_3},Br{F_3}\]
B. \[{S_2}{F_2},{S_2}C{l_2}\]
C. \[{O_2}{F_2},{S_2}C{l_2}\]
D. \[{B_2}{H_6},{N_2}{H_4}\]
Answer
576.6k+ views
Hint: \[{S_2}{F_2}\] and \[{S_2}C{l_2}\] both have \[s{p^3}d\] hybridization and has the shape which is similar to \[{H_2}{O_2}\]. In the same way, \[{O_2}{F_2}\] has the structure similar to \[{H_2}{O_2}\].
Complete step by step answer:
\[B{F_3}\] have the \[s{p^2}\] hybridization but \[Br{F_3}\] (Bromine Trifluoride) have the \[s{p^3}d\] hybridization. So, \[B{F_3}\] is triangular (Trigonal planar) in shape having bond angle \[120^\circ \] but \[Br{F_3}\] is ‘T’ shaped or trigonal bipyramidal with a bond angle of \[86.2^\circ \].

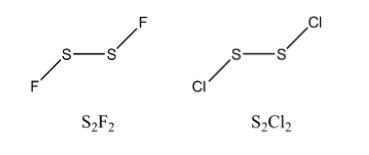
\[{S_2}{F_2}\] (Disulphuric difluoride) is a halide of sulphur have the \[s{p^3}{d^{}}\] hybridization. Its shape is gauche (half-open book shape) which is similar to \[{H_2}{O_2}\] with bond angle of \[108.3^\circ .{S_2}C{l_2}\] is a polar molecule and has \[s{p^3}d\] hybridization. It has the shape of gauche.
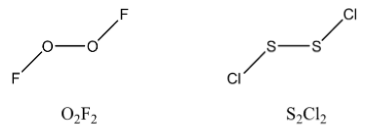
\[{O_2}{F_2}\] has the structure similar to \[{H_2}{O_2}\]. It has the shape of a half-open book (gauche in shape) \[{S_2}C{l_2}\] also has the shape similar to \[{O_2}{F_2}\].
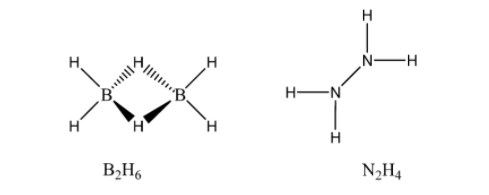
In (Diborane), Boron is \[s{p^3}\] hybridised, there are two terminals \[B - H\] bonds for each boron atom and there are only 12 bonding electrons available. The structure of Diborane molecule consists of 4 H atoms and that of two boron atoms coming on the same plane. In between these planes, there are said to be two dividing atoms of H. The two atoms of B left with that of each unpaired electron orbital and empty orbital forms the two bridging’s \[\left( {B-H-B} \right)\] bonds with that of the two 1s hydrogen atoms, is also called as the banana bond and on the other hand, \[{N_2}{H_4}\] (Nitrogen hydride or Hydrazine) is a strong base. Each subunit of \[{H_2}N - N\] is pyramidal and \[N - N\] bond distance is about \[1.4A^\circ \].
Therefore, the correct answer is option (B) and (C).
Note: Diborane is a chemical compound that consists of boron (B) and hydrogen (H) atoms and has a molecular formula \[{B_2}{H_6}\]. This substance is highly unstable at the room temperature and has a sweet odour. The compounds consisting of boron and hydrogen atoms are called boranes and diborane is one of the simplest boron hydrides.
Complete step by step answer:
\[B{F_3}\] have the \[s{p^2}\] hybridization but \[Br{F_3}\] (Bromine Trifluoride) have the \[s{p^3}d\] hybridization. So, \[B{F_3}\] is triangular (Trigonal planar) in shape having bond angle \[120^\circ \] but \[Br{F_3}\] is ‘T’ shaped or trigonal bipyramidal with a bond angle of \[86.2^\circ \].

\[{S_2}{F_2}\] (Disulphuric difluoride) is a halide of sulphur have the \[s{p^3}{d^{}}\] hybridization. Its shape is gauche (half-open book shape) which is similar to \[{H_2}{O_2}\] with bond angle of \[108.3^\circ .{S_2}C{l_2}\] is a polar molecule and has \[s{p^3}d\] hybridization. It has the shape of gauche.

\[{O_2}{F_2}\] has the structure similar to \[{H_2}{O_2}\]. It has the shape of a half-open book (gauche in shape) \[{S_2}C{l_2}\] also has the shape similar to \[{O_2}{F_2}\].

In (Diborane), Boron is \[s{p^3}\] hybridised, there are two terminals \[B - H\] bonds for each boron atom and there are only 12 bonding electrons available. The structure of Diborane molecule consists of 4 H atoms and that of two boron atoms coming on the same plane. In between these planes, there are said to be two dividing atoms of H. The two atoms of B left with that of each unpaired electron orbital and empty orbital forms the two bridging’s \[\left( {B-H-B} \right)\] bonds with that of the two 1s hydrogen atoms, is also called as the banana bond and on the other hand, \[{N_2}{H_4}\] (Nitrogen hydride or Hydrazine) is a strong base. Each subunit of \[{H_2}N - N\] is pyramidal and \[N - N\] bond distance is about \[1.4A^\circ \].

Therefore, the correct answer is option (B) and (C).
Note: Diborane is a chemical compound that consists of boron (B) and hydrogen (H) atoms and has a molecular formula \[{B_2}{H_6}\]. This substance is highly unstable at the room temperature and has a sweet odour. The compounds consisting of boron and hydrogen atoms are called boranes and diborane is one of the simplest boron hydrides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




