
The pair is known as:
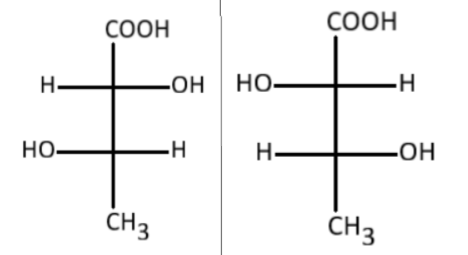
a) Structural isomers
b) Geometrical isomers
c) erythro stereoisomers
d) threo stereoisomers

Answer
573k+ views
Hint:Notice carefully the two figures are mirror images of each other, just like your hands. Also, the two figures differ in the spatial arrangement of atoms hence they are some kind of stereoisomers.
Complete answer:
Since they are stereoisomers therefore we can eliminate the option ‘a’ and we are left with 3 options.
It is also not a geometrical isomer, since there is no carbon-carbon double bond present in the two figures.Therefore, we are left with threo and stereoisomerism. Let's understand each of these in detail.
In a threo stereoisomer, the identical groups are present at the opposite sides of Fischer projection while in an erythro stereoisomer, the identical groups are present on the same side of Fischer projection.
Here, we are referring to the two -OH groups, and H atoms as identical pairs.
As the identical pairs are present at opposite sides
Therefore, the answer to this question is option (d) i.e., Threo stereoisomers.
Additional information:
1. In stereoisomerism, the molecules have the same molecular formula and sequence of the bonded atom, but they differ in the 3-dimensional orientation of the atoms in space.
2. Fischer projection is a 2-dimensional representation of a 3-dimensional organic molecule. It was devised by Emil Fischer in 1891
Note:
Proper knowledge of stereoisomerism is required otherwise it may lead to confusion bet threo stereoisomers,erythro stereoisomers and cis-trans isomers.
Complete answer:
Since they are stereoisomers therefore we can eliminate the option ‘a’ and we are left with 3 options.
It is also not a geometrical isomer, since there is no carbon-carbon double bond present in the two figures.Therefore, we are left with threo and stereoisomerism. Let's understand each of these in detail.
In a threo stereoisomer, the identical groups are present at the opposite sides of Fischer projection while in an erythro stereoisomer, the identical groups are present on the same side of Fischer projection.
Here, we are referring to the two -OH groups, and H atoms as identical pairs.
As the identical pairs are present at opposite sides
Therefore, the answer to this question is option (d) i.e., Threo stereoisomers.
Additional information:
1. In stereoisomerism, the molecules have the same molecular formula and sequence of the bonded atom, but they differ in the 3-dimensional orientation of the atoms in space.
2. Fischer projection is a 2-dimensional representation of a 3-dimensional organic molecule. It was devised by Emil Fischer in 1891
Note:
Proper knowledge of stereoisomerism is required otherwise it may lead to confusion bet threo stereoisomers,erythro stereoisomers and cis-trans isomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




