
The oxidation state of oxygen in$\text{ Cr}{{\text{O}}_{\text{5}}}$is:
A) $-1$
B) $-2$
C) Both A and B
D)$-\frac{1}{2}$
Answer
588k+ views
Hint: Here, the $\text{Cr}$ is bonded to the four peroxy oxygen and one coordinate oxygen thus the oxidation state of each oxygen is different. The$\text{ Cr}{{\text{O}}_{\text{5}}}$ is a butterfly-like structure. The oxidation state of chromium is $+6$ as it forms 6 bonds with the neighbouring oxygen atoms.
Complete step by step answer:
There are certain guidelines to determine the oxidation number of the element. The oxidation number is either positive or negative.
1) The atoms which exist in its elemental form have zero oxidation number.
2) Elements which exist as a single atom or monoatomic have oxidation number equal to their charge.
3) In a species, the sum of the oxidation number of all the atoms is equal to the charge on the species.
4) Net charge on the neutral molecules is always equal to zero.
Now, we can find out the oxidation state of sodium $\text{ O}$ in$\text{ Cr}{{\text{O}}_{\text{5}}}$.
$\text{ Cr}{{\text{O}}_{\text{5}}}$ Is a compound in which one chromium atom is bonded to four peroxy oxygen and on coordinated oxygen. The structure $\text{ Cr}{{\text{O}}_{\text{5}}}$is as shown below,
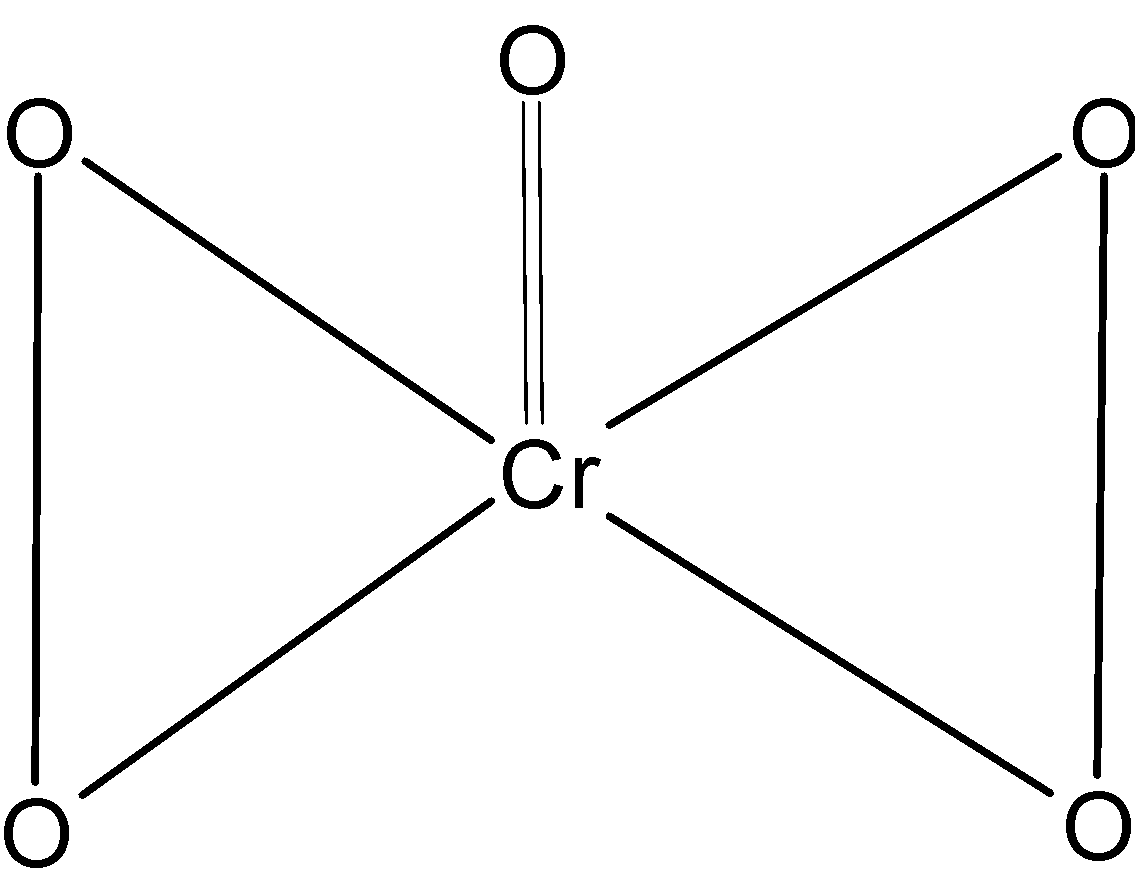
It is a ‘butterfly’ like structure where two four peroxy oxygens are on the opposite side of the chromium and one coordinate oxygen forming a double bond is at the top. Now we know that oxygen is electronegative than the chromium thus the oxidation state of coordinate oxygen is $-2$ . It is also normal oxygen.
IN $\text{ Cr}{{\text{O}}_{\text{5}}}$, the chromium $\text{ Cr}$ is forming 6 bonds with the oxygens. Since it donates its 6 electrons the oxidation state of chromium can be considered as the$+6$.
Now, we have the oxidation state of chromium and coordinate oxygen. We can determine the oxidation state of peroxy oxygen.
The net charge on the $\text{ Cr}{{\text{O}}_{\text{5}}}$ is zero. Therefore, The sum of the oxidation state of all species in the $\text{ Cr}{{\text{O}}_{\text{5}}}$ will be equal to zero.
$\begin{align}
& \text{O}\text{.S}\text{. of Cr}{{\text{O}}_{\text{5}}}\text{ = O}\text{.S}\text{. of Cr + O}\text{.S}\text{. of Coordinate Oxygen + 4 (O}\text{.S}\text{. of peroxy Oxygen)} \\
& \text{ 0 = +6 + (-2) + 4(x)} \\
& \Rightarrow \text{ 0 = +4 + 4(x)} \\
& \Rightarrow \text{ -4 = 4(x)} \\
& \Rightarrow \frac{-4}{4}\text{ = x} \\
& \therefore \text{ x = -1} \\
\end{align}$
That is the oxidation state of peroxy oxygen is $\text{-1}$.
Here, the oxidation state of oxygen in$\text{ Cr}{{\text{O}}_{\text{5}}}$ is $-2\text{ and }-\text{1}$ .
Hence, (C) is the correct option.
Note: The peroxy linkage is a very special type of linkage. The two oxygen atoms form a bond between them. One of the common examples of peroxy linkage is hydrogen peroxide${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$. The structure of it is,\[\text{ H}-\text{O}-\text{O}-\text{H}\]. In solving such a question try to figure out the structure of the given compound.
Complete step by step answer:
There are certain guidelines to determine the oxidation number of the element. The oxidation number is either positive or negative.
1) The atoms which exist in its elemental form have zero oxidation number.
2) Elements which exist as a single atom or monoatomic have oxidation number equal to their charge.
3) In a species, the sum of the oxidation number of all the atoms is equal to the charge on the species.
4) Net charge on the neutral molecules is always equal to zero.
Now, we can find out the oxidation state of sodium $\text{ O}$ in$\text{ Cr}{{\text{O}}_{\text{5}}}$.
$\text{ Cr}{{\text{O}}_{\text{5}}}$ Is a compound in which one chromium atom is bonded to four peroxy oxygen and on coordinated oxygen. The structure $\text{ Cr}{{\text{O}}_{\text{5}}}$is as shown below,

It is a ‘butterfly’ like structure where two four peroxy oxygens are on the opposite side of the chromium and one coordinate oxygen forming a double bond is at the top. Now we know that oxygen is electronegative than the chromium thus the oxidation state of coordinate oxygen is $-2$ . It is also normal oxygen.
IN $\text{ Cr}{{\text{O}}_{\text{5}}}$, the chromium $\text{ Cr}$ is forming 6 bonds with the oxygens. Since it donates its 6 electrons the oxidation state of chromium can be considered as the$+6$.
Now, we have the oxidation state of chromium and coordinate oxygen. We can determine the oxidation state of peroxy oxygen.
The net charge on the $\text{ Cr}{{\text{O}}_{\text{5}}}$ is zero. Therefore, The sum of the oxidation state of all species in the $\text{ Cr}{{\text{O}}_{\text{5}}}$ will be equal to zero.
$\begin{align}
& \text{O}\text{.S}\text{. of Cr}{{\text{O}}_{\text{5}}}\text{ = O}\text{.S}\text{. of Cr + O}\text{.S}\text{. of Coordinate Oxygen + 4 (O}\text{.S}\text{. of peroxy Oxygen)} \\
& \text{ 0 = +6 + (-2) + 4(x)} \\
& \Rightarrow \text{ 0 = +4 + 4(x)} \\
& \Rightarrow \text{ -4 = 4(x)} \\
& \Rightarrow \frac{-4}{4}\text{ = x} \\
& \therefore \text{ x = -1} \\
\end{align}$
That is the oxidation state of peroxy oxygen is $\text{-1}$.
Here, the oxidation state of oxygen in$\text{ Cr}{{\text{O}}_{\text{5}}}$ is $-2\text{ and }-\text{1}$ .
Hence, (C) is the correct option.
Note: The peroxy linkage is a very special type of linkage. The two oxygen atoms form a bond between them. One of the common examples of peroxy linkage is hydrogen peroxide${{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$. The structure of it is,\[\text{ H}-\text{O}-\text{O}-\text{H}\]. In solving such a question try to figure out the structure of the given compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




