
The order of stability of carbanion is:
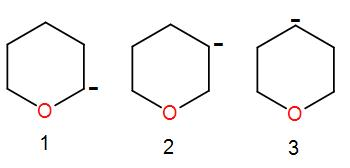
[A] $1>2>3$
[B] $2>3>1$
[C] $3>2>1$
[D] None of these

Answer
588.9k+ views
Hint: Stability of a carbanion depends upon resonance, hybridisation and inductive effect. To solve this check if the structures are resonance stabilised or not and check if the hybridisation of the carbon atom is different. Remember that inductive effect dies with distance. Identify the factors applicable here to solve this question.
Complete step by step answer:
Before answering the question, let us understand what a carbanion is. It is basically an anion of carbon (i.e. the carbon bears a negative charge) where the carbon has an unshared pair of electrons.
Now let us discuss the factors that affect the stability of a carbanion.
- The most important factor that makes a carbanion stable (generally in an aromatic ring) is resonance. If it is resonance stabilised, it is highly stable. Higher the resonating structures higher will be the stability.
- Another important factor for stability is inductive effect. If an electronegative atom is present beside the anion then it provides greater stability.
- The hybridisation of the charge bearing atoms also plays an important role in its stability. Higher the s-character, higher is the stability of the carbanion.
Now, let us discuss the question given to us.
We can see that the systems given to us are not conjugated and thus there is no possibility of resonance here. So the resonance factor will not play any role here.
Also we can see that the hybridisation will also remain the same in each case so hybridisation is not the factor that we need to consider.
Lastly, we have an inductive effect. We know that inductive effect decreases with distance.
In the first diagram, the negative charge is closest to the electronegative atom and thus it will have the highest inductive effect. Therefore, this is the most stable carbanion followed by 2 and 3.
So, the order of stability of carbanion is $1 > 2 > 3$.
So, the correct answer is “Option A”.
Note: We should remember that –I effect stabilize a carbanion but +I effect stabilize a carbanion. To denote the p-orbitals, we use the symbol $\omega $ (lower case omega). A carbanion has 2 electrons thus we denote it as $_{\omega }2$ and we denote a carbocation as $_{\omega }0$ because it has 0 electrons. However, if the two new bonds are formed to the same lobe of the p-orbital of the carbanion, we have a $_{\omega }{{2}_{s}}$ component and when we have the new bonds on different loves, we have a $_{\omega }{{2}_{a}}$ component.
Complete step by step answer:
Before answering the question, let us understand what a carbanion is. It is basically an anion of carbon (i.e. the carbon bears a negative charge) where the carbon has an unshared pair of electrons.
Now let us discuss the factors that affect the stability of a carbanion.
- The most important factor that makes a carbanion stable (generally in an aromatic ring) is resonance. If it is resonance stabilised, it is highly stable. Higher the resonating structures higher will be the stability.
- Another important factor for stability is inductive effect. If an electronegative atom is present beside the anion then it provides greater stability.
- The hybridisation of the charge bearing atoms also plays an important role in its stability. Higher the s-character, higher is the stability of the carbanion.
Now, let us discuss the question given to us.
We can see that the systems given to us are not conjugated and thus there is no possibility of resonance here. So the resonance factor will not play any role here.
Also we can see that the hybridisation will also remain the same in each case so hybridisation is not the factor that we need to consider.
Lastly, we have an inductive effect. We know that inductive effect decreases with distance.
In the first diagram, the negative charge is closest to the electronegative atom and thus it will have the highest inductive effect. Therefore, this is the most stable carbanion followed by 2 and 3.
So, the order of stability of carbanion is $1 > 2 > 3$.
So, the correct answer is “Option A”.
Note: We should remember that –I effect stabilize a carbanion but +I effect stabilize a carbanion. To denote the p-orbitals, we use the symbol $\omega $ (lower case omega). A carbanion has 2 electrons thus we denote it as $_{\omega }2$ and we denote a carbocation as $_{\omega }0$ because it has 0 electrons. However, if the two new bonds are formed to the same lobe of the p-orbital of the carbanion, we have a $_{\omega }{{2}_{s}}$ component and when we have the new bonds on different loves, we have a $_{\omega }{{2}_{a}}$ component.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




