
The optically active molecule is:
A)
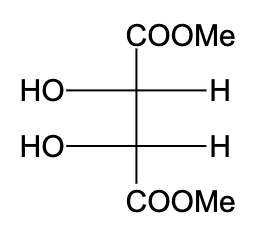
B)
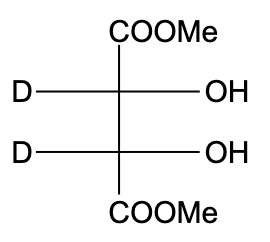
C)

D)





Answer
548.4k+ views
Hint: Optical isomers are called enantiomers. They have identical chemical as well as physical properties. The Optical isomerism is associated with a plane of symmetry of the compound.
Complete step by step answer:
- Molecules which are optical isomers are known as enantiomers. They have identical chemical and physical properties. Only their effect on the plane of polarised light and reaction with the other chiral molecules are not the same.
- A chiral molecule that rotates through a plane of polarised light is said to be optically active. If the molecule rotates in a clockwise direction, we call that rotation a dextrorotatory rotation. However, if the light rotates counter-clockwise, we call that rotation as levorotatory.
- To identify the optically active molecule given in the question, we will consider the plane of symmetry of the compound. If there is an element of the plane of symmetry, the compound is optically inactive, if not found, and then it is optically active.
-In option (A), the compound is not optically active due to the presence of a plane of symmetry.

- In option (B), the compound is not optically active due to the presence of a plane of symmetry
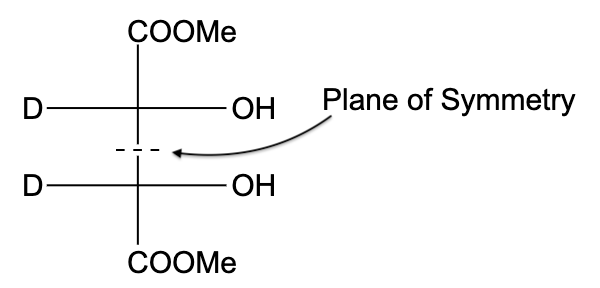
- In option (C), the compound is optically active, due to the presence of a plane of symmetry.
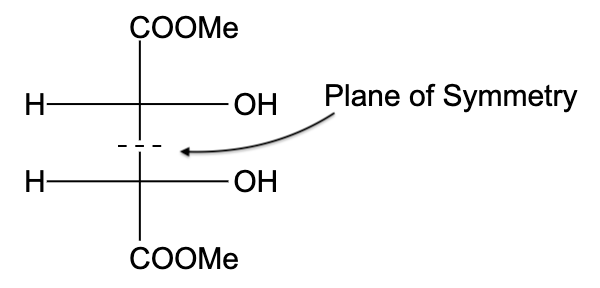
- In option (D), the compound is not optically active due to the presence of a plane of symmetry
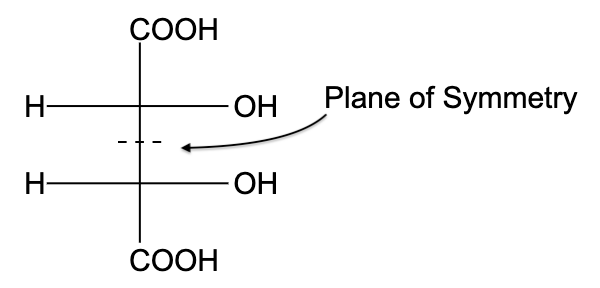
So, the correct answer is “Option C”.
Note: Chiral molecules are optically active. Therefore, when a beam of plane-polarized light passes through a chiral molecule, it interacts with the molecule in such a way that the angle of the plane of oscillation rotates.
Complete step by step answer:
- Molecules which are optical isomers are known as enantiomers. They have identical chemical and physical properties. Only their effect on the plane of polarised light and reaction with the other chiral molecules are not the same.
- A chiral molecule that rotates through a plane of polarised light is said to be optically active. If the molecule rotates in a clockwise direction, we call that rotation a dextrorotatory rotation. However, if the light rotates counter-clockwise, we call that rotation as levorotatory.
- To identify the optically active molecule given in the question, we will consider the plane of symmetry of the compound. If there is an element of the plane of symmetry, the compound is optically inactive, if not found, and then it is optically active.
-In option (A), the compound is not optically active due to the presence of a plane of symmetry.

- In option (B), the compound is not optically active due to the presence of a plane of symmetry

- In option (C), the compound is optically active, due to the presence of a plane of symmetry.

- In option (D), the compound is not optically active due to the presence of a plane of symmetry

So, the correct answer is “Option C”.
Note: Chiral molecules are optically active. Therefore, when a beam of plane-polarized light passes through a chiral molecule, it interacts with the molecule in such a way that the angle of the plane of oscillation rotates.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




