
The one that will show optical activity is:
(en = ethane-1, 2-diamine)
(A)
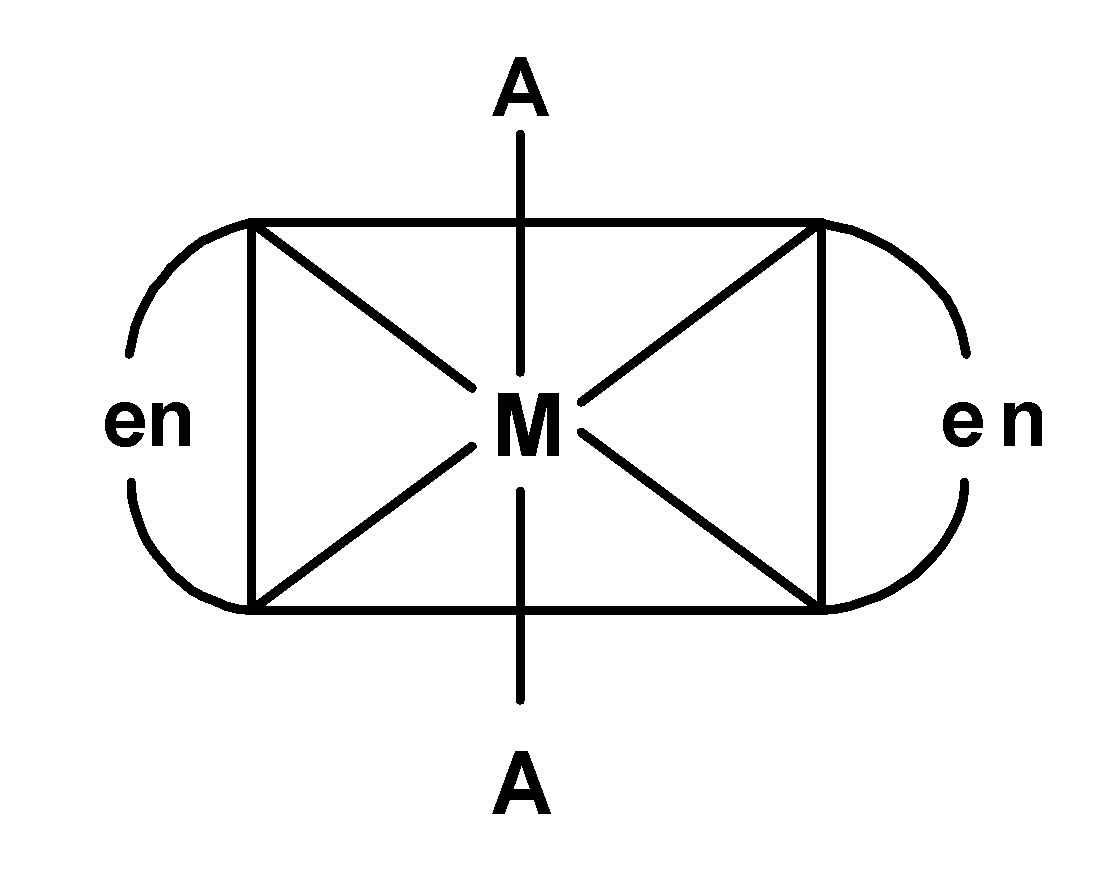
(B)
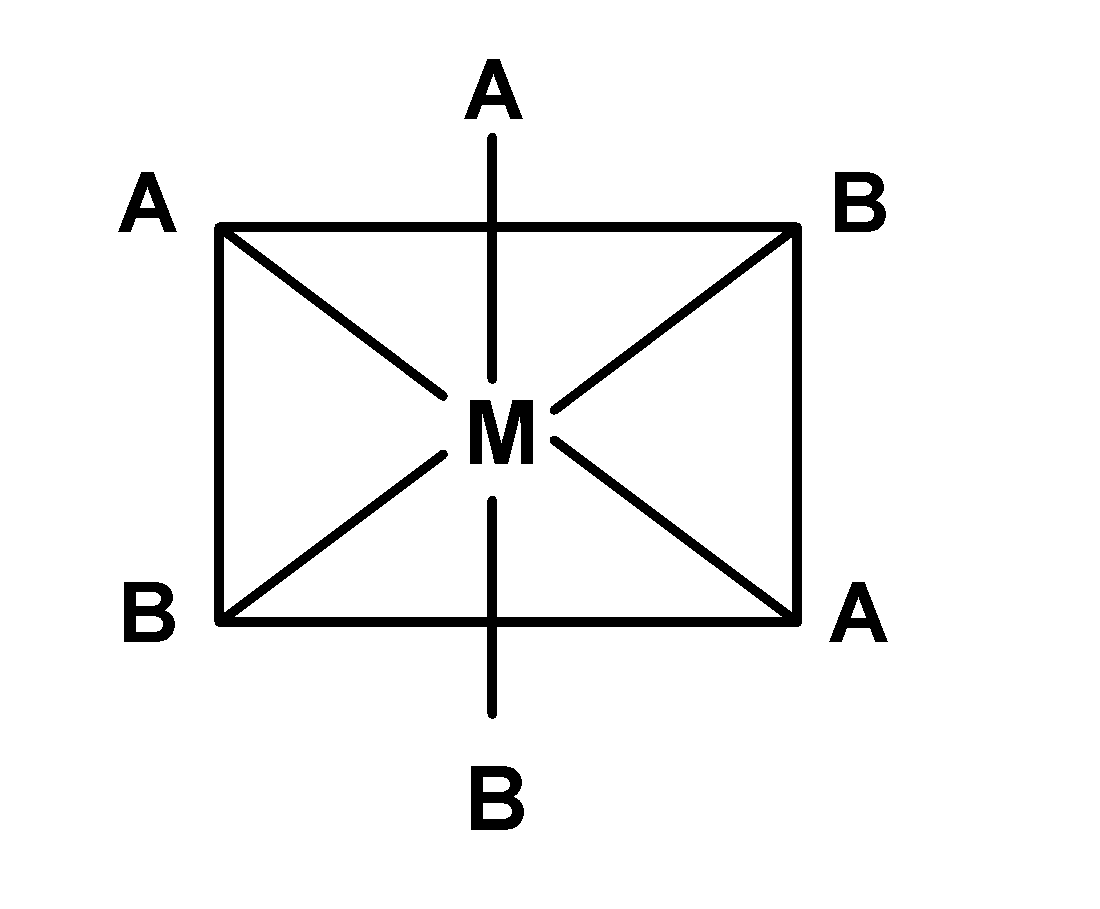
(C)
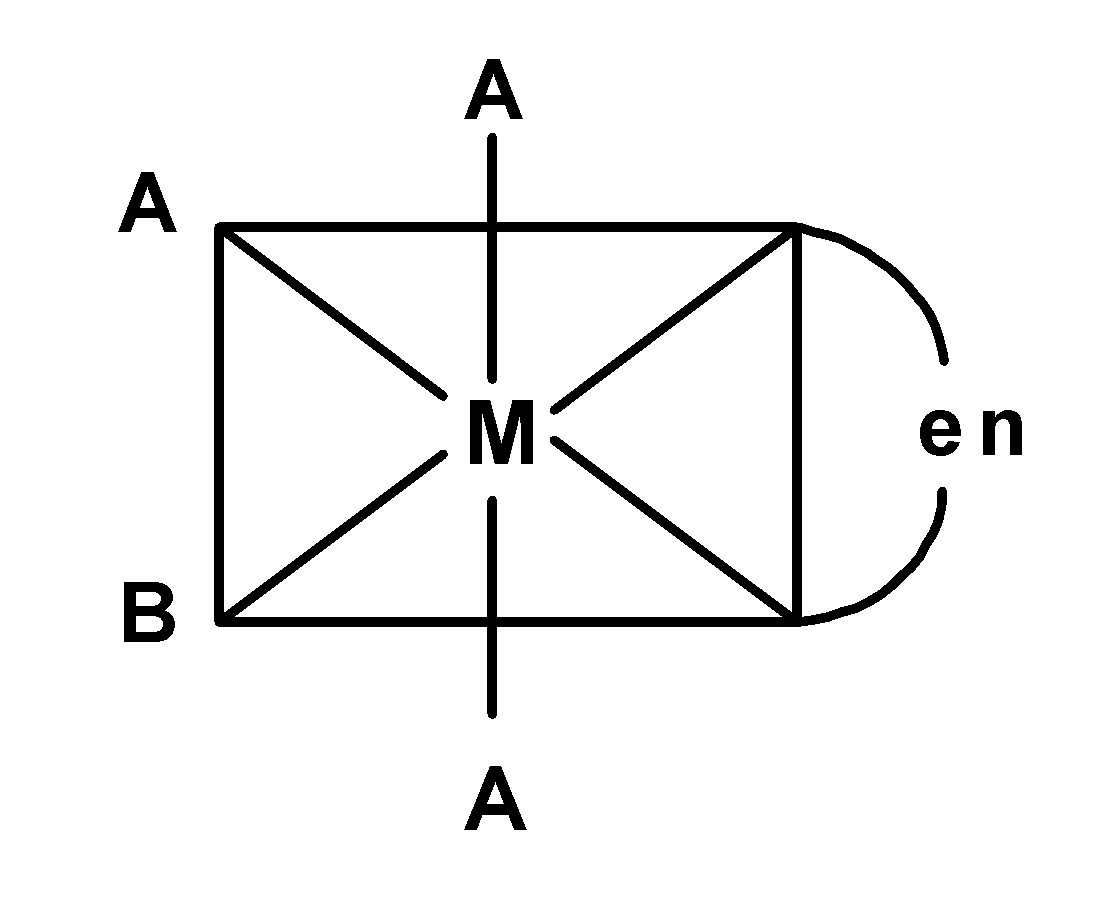
(D)
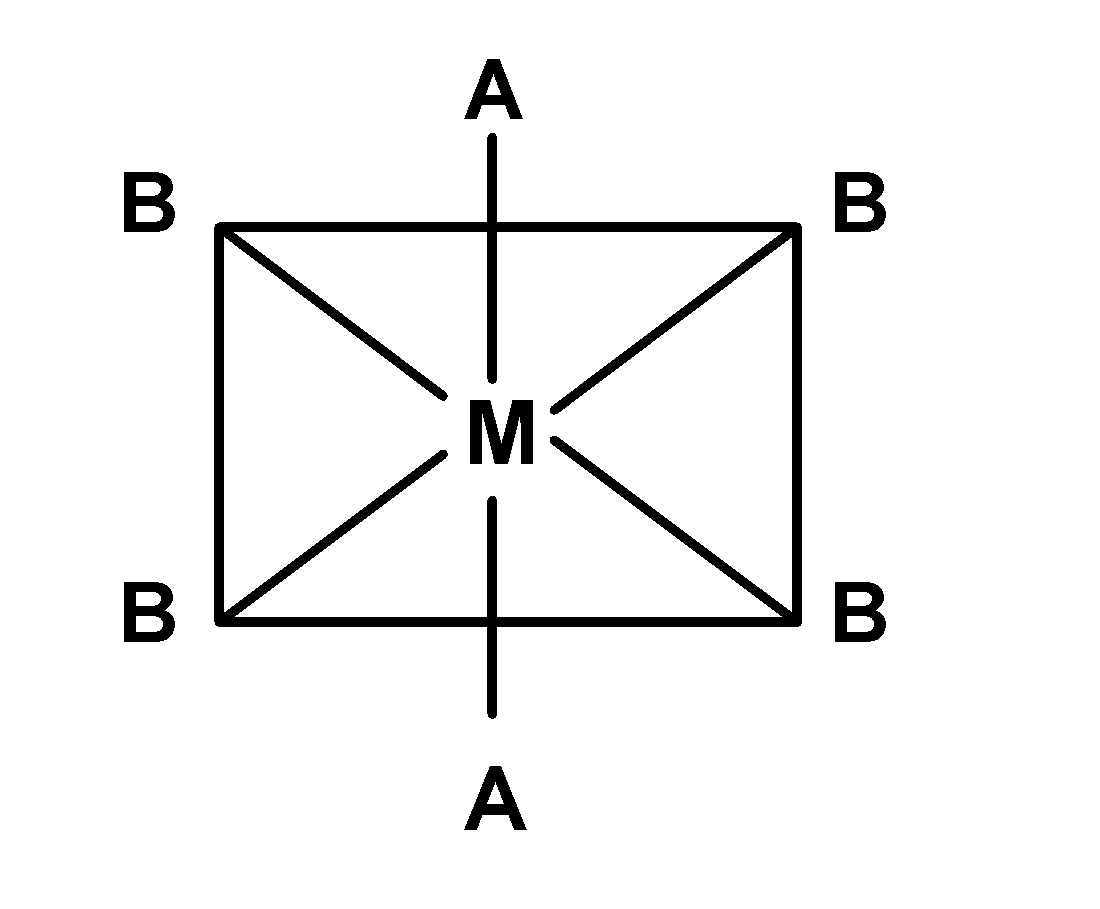
| (A) | 
|
| (B) | 
|
| (C) | 
|
| (D) | 
|
Answer
578.7k+ views
Hint: The isomers which can rotate the plane of polarised light are known as the optically active complexes. The essential condition for the complex to exhibit the optical activity is that the isomer should not possess the plane of symmetry in the structure. If the plane divides the complex into the equal half then the complex is optically inactive. However, if not then the complex is said to be optically active. The bidentate ligand donates its two-electrons and forms a bond with the metal.
Complete step by step answer:
-Stereoisomers are those isomers that have the same position as atoms or groups but they differ in the spatial arrangement around the central atom.
-There is a certain substance that can rotate the plane polarised light. These are called as the optically active substance. The isomers which rotate the plane of polarised light equally but in opposite directions are called the optically active isomers.
-The essential condition for a substance to show optical activity is that the substance should not have a plane of symmetry in its structure. The optical isomers have identical physical and chemical properties. They differ only in the direction in which they rotate the plane of polarised light.
-The complexes which have the $\text{ }\left[ \text{M(AA)}{{\text{X}}_{\text{2}}}{{\text{Y}}_{\text{2}}} \right]\text{ }$formula shows the optical activity. Here, M is the central metal atom, (AA) is a bidentate ligand, and X and Y are other ligands.
-The general representation of the $\text{ }\left[ \text{M(AA)}{{\text{X}}_{\text{2}}}{{\text{Y}}_{\text{2}}} \right]\text{ }$type structure is as shown below,
-The complex contains one symmetrical bidentate ligand such as ethane-1, 2-diamine do not possess the plane of symmetry. The mirror image and the complex are non-superimposable mirror images thus, the $\text{ }\left[ \text{M(en)}{{\text{X}}_{\text{2}}}{{\text{Y}}_{\text{2}}} \right]\text{ }$are an optically active complex.
-Let's take the example of a complex, where the central metal atom is cobalt surrounded by one ethane-1, 2-diamine ligand, two chloride ligand, and two amine ligand. The structure is as shown below, $\text{ }{{\left[ \text{Co(en)(N}{{\text{H}}_{3}}{{\text{)}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}} \right]}^{+}}\text{ }$
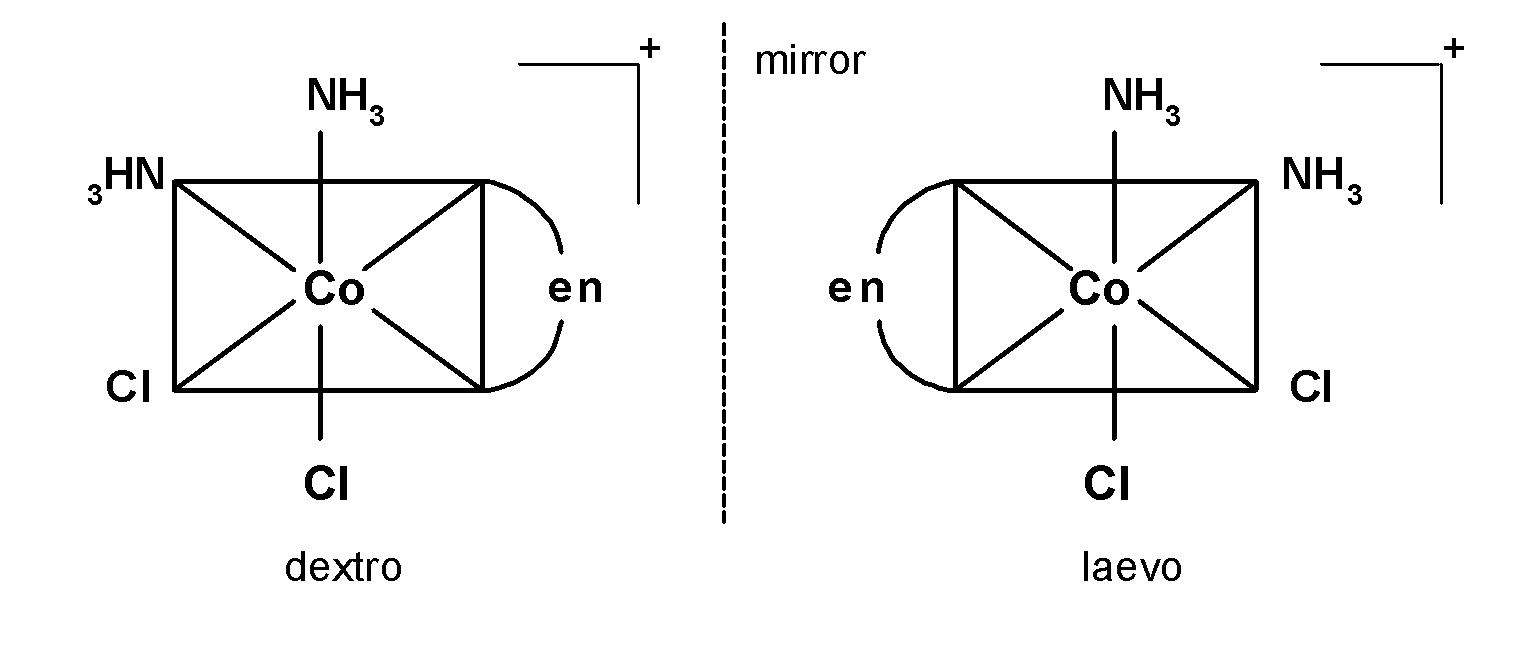
-In A) complex, the plane of symmetry passes through $\text{ A-M-A }$ thus the complex $\text{ }\left[ \text{M(AA}{{\text{)}}_{\text{2}}}{{\text{A}}_{\text{2}}} \right]\text{ }$ is optically inactive. the similar is applicable for the complex D).
-In complex B), the plane of symmetry passes diagonally through the complex. Thus it is optically inactive. The compound $\text{ }\left[ \text{M(en)}{{\text{X}}_{\text{2}}}{{\text{Y}}_{\text{2}}} \right]\text{ }$is optically active.
Hence, (C) is the correct option.
Note: Note that, apart from $\text{ }\left[ \text{M(AA)}{{\text{X}}_{\text{2}}}{{\text{Y}}_{\text{2}}} \right]\text{ }$there are some other complexes which show the optical activity. This has the general structure as,
1. $\text{ }\left[ \text{M(AA}{{\text{)}}_{\text{3}}} \right]\text{ }$(Example,$\text{ }{{\left[ \text{Co(en}{{\text{)}}_{\text{3}}} \right]}^{\text{3+}}}\text{ }$)
2. $\text{ }\left[ \text{M(AA}{{\text{)}}_{2}}{{\text{X}}_{\text{2}}} \right]\text{ }$(Example,$\text{ }{{\left[ \text{M(en}{{\text{)}}_{2}}\text{C}{{\text{l}}_{\text{2}}} \right]}^{+}}\text{ }$)
3. Octahedral complexes with hexadentate ligands like EDTA show optical isomerism.
Complete step by step answer:
-Stereoisomers are those isomers that have the same position as atoms or groups but they differ in the spatial arrangement around the central atom.
-There is a certain substance that can rotate the plane polarised light. These are called as the optically active substance. The isomers which rotate the plane of polarised light equally but in opposite directions are called the optically active isomers.
-The essential condition for a substance to show optical activity is that the substance should not have a plane of symmetry in its structure. The optical isomers have identical physical and chemical properties. They differ only in the direction in which they rotate the plane of polarised light.
-The complexes which have the $\text{ }\left[ \text{M(AA)}{{\text{X}}_{\text{2}}}{{\text{Y}}_{\text{2}}} \right]\text{ }$formula shows the optical activity. Here, M is the central metal atom, (AA) is a bidentate ligand, and X and Y are other ligands.
-The general representation of the $\text{ }\left[ \text{M(AA)}{{\text{X}}_{\text{2}}}{{\text{Y}}_{\text{2}}} \right]\text{ }$type structure is as shown below,
-The complex contains one symmetrical bidentate ligand such as ethane-1, 2-diamine do not possess the plane of symmetry. The mirror image and the complex are non-superimposable mirror images thus, the $\text{ }\left[ \text{M(en)}{{\text{X}}_{\text{2}}}{{\text{Y}}_{\text{2}}} \right]\text{ }$are an optically active complex.
-Let's take the example of a complex, where the central metal atom is cobalt surrounded by one ethane-1, 2-diamine ligand, two chloride ligand, and two amine ligand. The structure is as shown below, $\text{ }{{\left[ \text{Co(en)(N}{{\text{H}}_{3}}{{\text{)}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}} \right]}^{+}}\text{ }$

-In A) complex, the plane of symmetry passes through $\text{ A-M-A }$ thus the complex $\text{ }\left[ \text{M(AA}{{\text{)}}_{\text{2}}}{{\text{A}}_{\text{2}}} \right]\text{ }$ is optically inactive. the similar is applicable for the complex D).
-In complex B), the plane of symmetry passes diagonally through the complex. Thus it is optically inactive. The compound $\text{ }\left[ \text{M(en)}{{\text{X}}_{\text{2}}}{{\text{Y}}_{\text{2}}} \right]\text{ }$is optically active.
Hence, (C) is the correct option.
Note: Note that, apart from $\text{ }\left[ \text{M(AA)}{{\text{X}}_{\text{2}}}{{\text{Y}}_{\text{2}}} \right]\text{ }$there are some other complexes which show the optical activity. This has the general structure as,
1. $\text{ }\left[ \text{M(AA}{{\text{)}}_{\text{3}}} \right]\text{ }$(Example,$\text{ }{{\left[ \text{Co(en}{{\text{)}}_{\text{3}}} \right]}^{\text{3+}}}\text{ }$)
2. $\text{ }\left[ \text{M(AA}{{\text{)}}_{2}}{{\text{X}}_{\text{2}}} \right]\text{ }$(Example,$\text{ }{{\left[ \text{M(en}{{\text{)}}_{2}}\text{C}{{\text{l}}_{\text{2}}} \right]}^{+}}\text{ }$)
3. Octahedral complexes with hexadentate ligands like EDTA show optical isomerism.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




