
The number of valence-shell bonding electron-dot model for $H{N_3}$ is:
A: $6$
B: $10$
C: $11$
D: $16$
Answer
588.9k+ views
Hint: $H{N_3}$ is hydrazoic acid. $H{N_3}$ is also known as hydrogen azide or azoimide. In this compound there is one atom of hydrogen and three atoms of nitrogen. Hydrazoic acid has resonating structures. Hydrazoic acid is soluble in water.
Complete step by step answer:
$H{N_3}$ is hydrazoic acid. $H{N_3}$ is also known as hydrogen azide or azoimide. In this question we have to find the number of valence shell bonding electrons in electron-dot structure. Electron dot structure is also called lewis dot structure or lewis structure. In electron-dot structure valence electrons are represented by dots around the atom. In the $H{N_3}$ molecule there is one atom of hydrogen and three atoms of nitrogen. This molecule has resonating structures. These structures are:
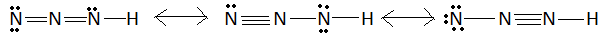
This is the structure of the $H{N_3}$ molecule. Corresponding electron dot structure is:

In this structure red dots represent the bonding electrons. Number of these red dots is equal to the number of valence shell bonding electrons. These red dots are $10$ in each structure. This means the number of valence shell bonding electrons for $H{N_3}$ molecules is $10$.
So, the correct answer is option B.
Additional information: Resonance structures are also called canonical structures. Resonating structures are the structures that belong to the same molecule. These structures are different from each other in terms of position of electrons. Position of atoms is the same in all the structures.
Note:
From the electron dot structure we can find both, the valence shell electron and number of bonding electrons. Like in this structure the sum of all the dots (red and black) for a structure gives the number of valence electrons and sum of only red dots gives the number of bonding electrons.
Complete step by step answer:
$H{N_3}$ is hydrazoic acid. $H{N_3}$ is also known as hydrogen azide or azoimide. In this question we have to find the number of valence shell bonding electrons in electron-dot structure. Electron dot structure is also called lewis dot structure or lewis structure. In electron-dot structure valence electrons are represented by dots around the atom. In the $H{N_3}$ molecule there is one atom of hydrogen and three atoms of nitrogen. This molecule has resonating structures. These structures are:
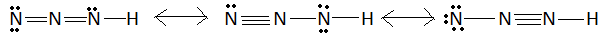
This is the structure of the $H{N_3}$ molecule. Corresponding electron dot structure is:

In this structure red dots represent the bonding electrons. Number of these red dots is equal to the number of valence shell bonding electrons. These red dots are $10$ in each structure. This means the number of valence shell bonding electrons for $H{N_3}$ molecules is $10$.
So, the correct answer is option B.
Additional information: Resonance structures are also called canonical structures. Resonating structures are the structures that belong to the same molecule. These structures are different from each other in terms of position of electrons. Position of atoms is the same in all the structures.
Note:
From the electron dot structure we can find both, the valence shell electron and number of bonding electrons. Like in this structure the sum of all the dots (red and black) for a structure gives the number of valence electrons and sum of only red dots gives the number of bonding electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




