
The number of unpaired electrons in nickel carbonyl is :
(A). Zero
(B). one
(C). four
(D). five
Answer
590.7k+ views
Hint: The number of unpaired electrons in nickel carbonyl depends on the complex formation, which further depends on multiple factors such as the electronic configuration of Ni and the influence of Co (whether it is a weak field or a strong field ligand).
Complete step by step answer:
We know that nickel carbonyl is also known as nickel tetracarbonyl $\left[ {Ni{{\left( {CO} \right)}_4}} \right]$ . It is a colorless liquid . So let us understand the structure and safety of nickel carbonyl . In nickel tetracarbonyl , nickel $\left( {Ni} \right)$ is $ + ve$ central atom with atomic number 28 and ground state electronic configuration $\left[ {{}_{18}Ar} \right]3{d^8}4{s^2}$ .
i.e
Ni :- Atomic number 28 :-
Ground state electronic configuration of valence shell .
$3{d^8}$ $4s$ $4p$
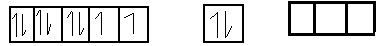
As carbon monoxide is the strong field legend and oxidation state 0 . When it approaches near nickel atom being strong field legend , it causes pairing of electron such that electrons of 4s orbital gets pair up with electrons to give valence shell configuration $\left[ {{}_{18}Ar} \right]3{d^{10}}4{s^0}$ .
So we gate 3 empty subshell in nickel atom i.e.
Orbital of 4s subshell and 3 subshell of 4p orbital . Now these for empty orbital undergo hybridization to form bonds with 4 C O atoms by accepting then subshell electrons.
Electronic configuration of $Ni{\left( {CO} \right)_4}$ .
Ni :- 28 $3{d^{10}}$ $4{s^2}$ $4{p^6}$
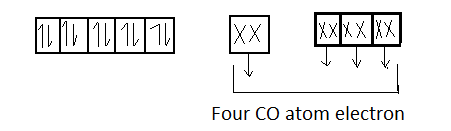
Since 1 orbital of subshell and 3 orbitals of p subshell undergo hybridization . So the hybridization of $Ni{\left( {CO} \right)_4}$ will be $s{p^3}$ its shape is tetrahedral . Moreover we see that there are no unpaired electrons present in $Ni{\left( {CO} \right)_4}$ , so it is a diamagnetic compound .
So the number of unpaired electrons in $Ni{\left( {CO} \right)_4}$ is zero .
So, the correct answer is Option A..
Note:
The legends which do not cause pairing of electrons of central atom are known has weak field legends and legend which cause pairing of ${e^{ - s}}$ are known as strong field legends the arrangement of the legend in increasing order of causing pairing strength are known has spectrochemical series .
${F^ - } < O{H^ - } < {C_2}O_4^{2 - } < {H_2}O < NC{S^ - } < edta < N{H_3} < en < C{N^ - } < CO$
Complete step by step answer:
We know that nickel carbonyl is also known as nickel tetracarbonyl $\left[ {Ni{{\left( {CO} \right)}_4}} \right]$ . It is a colorless liquid . So let us understand the structure and safety of nickel carbonyl . In nickel tetracarbonyl , nickel $\left( {Ni} \right)$ is $ + ve$ central atom with atomic number 28 and ground state electronic configuration $\left[ {{}_{18}Ar} \right]3{d^8}4{s^2}$ .
i.e
Ni :- Atomic number 28 :-
Ground state electronic configuration of valence shell .
$3{d^8}$ $4s$ $4p$

As carbon monoxide is the strong field legend and oxidation state 0 . When it approaches near nickel atom being strong field legend , it causes pairing of electron such that electrons of 4s orbital gets pair up with electrons to give valence shell configuration $\left[ {{}_{18}Ar} \right]3{d^{10}}4{s^0}$ .
So we gate 3 empty subshell in nickel atom i.e.
Orbital of 4s subshell and 3 subshell of 4p orbital . Now these for empty orbital undergo hybridization to form bonds with 4 C O atoms by accepting then subshell electrons.
Electronic configuration of $Ni{\left( {CO} \right)_4}$ .
Ni :- 28 $3{d^{10}}$ $4{s^2}$ $4{p^6}$

Since 1 orbital of subshell and 3 orbitals of p subshell undergo hybridization . So the hybridization of $Ni{\left( {CO} \right)_4}$ will be $s{p^3}$ its shape is tetrahedral . Moreover we see that there are no unpaired electrons present in $Ni{\left( {CO} \right)_4}$ , so it is a diamagnetic compound .
So the number of unpaired electrons in $Ni{\left( {CO} \right)_4}$ is zero .
So, the correct answer is Option A..
Note:
The legends which do not cause pairing of electrons of central atom are known has weak field legends and legend which cause pairing of ${e^{ - s}}$ are known as strong field legends the arrangement of the legend in increasing order of causing pairing strength are known has spectrochemical series .
${F^ - } < O{H^ - } < {C_2}O_4^{2 - } < {H_2}O < NC{S^ - } < edta < N{H_3} < en < C{N^ - } < CO$
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE




