
The number of s-s bonds in polythionic acid ${{\text{H}}_{\text{2}}}{{\text{S}}_{\text{n}}}{{\text{O}}_{\text{6}}}$ ?
A. \[{\text{n}}\]
B. \[{\text{n - 1}}\]
C. \[{\text{n - 2}}\]
D. None of these
Answer
574.8k+ views
Hint: Polythionic acid is an oxoacid which has a straight chain of sulfur atoms and has the chemical formula ${{\text{S}}_{\text{n}}}{{\text{(S}}{{\text{O}}_{\text{3}}}{\text{H)}}_{\text{2}}}{\text{ (n > 2)}}$. Trithionic acid \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{3}}}{{\text{O}}_{\text{6}}}\] , tetrathionic acid \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{4}}}{{\text{O}}_{\text{6}}}\] are simple examples. They are the conjugate acids of polythionates. Dithionic acid \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{6}}}\] does not belong to the polythionic acids due to strongly different properties.
Complete step by step solution:
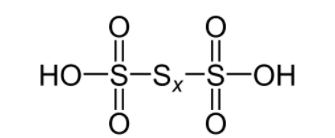
The number of \[{\text{s - s}}\] bonds in polythionic acid \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{n}}}{{\text{O}}_{\text{6}}}\] are \[{\text{n - 1}}\]
As we can see from the diagram, number of \[{\text{s - s}}\] bonds in polythionic acid is equal to \[{\text{n - 1}}\]
Hence, option “B” is correct
Additional Information
Any of a series of unstable acids containing a short chain of sulphur atoms, and having the general formula \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{n}}}{{\text{O}}_{\text{6}}}\] where n is \[2,{\text{ }}3,{\text{ }}4,\] or more are named as "dithionic", "trithionic", "tetrathionic", "pentathionic" acid respectively.
Note:
1. All polythionates anion contains chains of sulfur atoms attached to the terminal \[{\text{S}}{{\text{O}}_{\text{3}}}{\text{H}}\] -groups. Names of polythionic acids are determined by the number of atoms in the chain of sulfur atoms:
2. Polythionic acids with a small number of sulfur atoms in the chain \[{\text{n = 3, 4, 5, 6}}\] are the most stable. Polythionic acids are stable only in aqueous solutions, and are rapidly destroyed at higher concentrations with the release of sulfur, sulfur dioxide and - sometimes - sulfuric acid.
Complete step by step solution:

The number of \[{\text{s - s}}\] bonds in polythionic acid \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{n}}}{{\text{O}}_{\text{6}}}\] are \[{\text{n - 1}}\]
As we can see from the diagram, number of \[{\text{s - s}}\] bonds in polythionic acid is equal to \[{\text{n - 1}}\]
Hence, option “B” is correct
Additional Information
Any of a series of unstable acids containing a short chain of sulphur atoms, and having the general formula \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{n}}}{{\text{O}}_{\text{6}}}\] where n is \[2,{\text{ }}3,{\text{ }}4,\] or more are named as "dithionic", "trithionic", "tetrathionic", "pentathionic" acid respectively.
Note:
1. All polythionates anion contains chains of sulfur atoms attached to the terminal \[{\text{S}}{{\text{O}}_{\text{3}}}{\text{H}}\] -groups. Names of polythionic acids are determined by the number of atoms in the chain of sulfur atoms:
2. Polythionic acids with a small number of sulfur atoms in the chain \[{\text{n = 3, 4, 5, 6}}\] are the most stable. Polythionic acids are stable only in aqueous solutions, and are rapidly destroyed at higher concentrations with the release of sulfur, sulfur dioxide and - sometimes - sulfuric acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




