
The number of S-S bond in sulphur trioxide trimer $\left( {{\text{S}}_{3}}{{\text{O}}_{9}} \right)$ is:
A. 3
B. 2
C. 1
D. 0
Answer
596.4k+ views
Hint: Sulphur is denoted by 'S'. The S-S bond shows the presence of a single bond only that means other bonds must be attached to the oxygen atom. Sulphur trioxide has a molecular formula $\text{S}{{\text{O}}_{3}}$ which is also a primary pollutant in the acid rain.
Complete Step-by-Step Answer:
-To calculate the number of bonds of S-S in the structure of sulphur trioxide trimer, firstly we have to draw the structure:
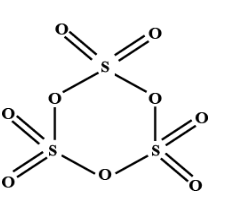
-In the structure, we can see that three molecules of sulphur are present and each sulphur is bonded with four oxygen atoms. Two oxygen are attached through the double bond and two oxygen atoms are attached through the single bond.
-So, we can clearly see that there is no bond between two sulphur atoms (S-S).
-Hence, option D. is the correct answer.
Additional Information:
-The smell of sulphur trioxide is very pungent in the vapour phase whereas the mist of sulphur trioxide is odourless.
-In the liquid and gaseous state, sulphur trioxide is colourless but in solid-state its colour varies from colourless to white crystalline.
-Sulphur trioxide is very hazardous for our health because while doing any experiment if anyone inhales or ingest it by mistake then it may cause serious burns.
-Sulphur trioxide is also very corrosive in nature.
Note: The disulphide bond is the common name of the S-S bond. The disulphide bond is mostly found in the protein structure example, in cysteine and in antibodies structure also. The net charge on the disulphide bond is -2.
Complete Step-by-Step Answer:
-To calculate the number of bonds of S-S in the structure of sulphur trioxide trimer, firstly we have to draw the structure:

-In the structure, we can see that three molecules of sulphur are present and each sulphur is bonded with four oxygen atoms. Two oxygen are attached through the double bond and two oxygen atoms are attached through the single bond.
-So, we can clearly see that there is no bond between two sulphur atoms (S-S).
-Hence, option D. is the correct answer.
Additional Information:
-The smell of sulphur trioxide is very pungent in the vapour phase whereas the mist of sulphur trioxide is odourless.
-In the liquid and gaseous state, sulphur trioxide is colourless but in solid-state its colour varies from colourless to white crystalline.
-Sulphur trioxide is very hazardous for our health because while doing any experiment if anyone inhales or ingest it by mistake then it may cause serious burns.
-Sulphur trioxide is also very corrosive in nature.
Note: The disulphide bond is the common name of the S-S bond. The disulphide bond is mostly found in the protein structure example, in cysteine and in antibodies structure also. The net charge on the disulphide bond is -2.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




